Thoracic surgery is widely associated with a great degree of unavoidable surgical injury and related to a high level of pain in the postoperative period. This has been proven in multiple studies and is well established in the scientific literature [1].
A combination of this fact and other factors such as an aging population, increasing number of comorbidities in patients presenting for elective surgery, a steadily growing shift towards less invasive, albeit sometimes longer and more complicated, surgical techniques [2], encourages anesthesiologists to seek improvements in anesthesia management to adjust accordingly and provide a safe and reliable patient service.
Tendency for less invasive surgical approach in thoracic surgery
In recent years video-assisted thoracoscopic surgery (VATS) has entered the mainstay of thoracic surgery and become a widely accepted technique for both pulmonary wedge resection and more complex procedures, including pulmonary lobectomy. It is associated with decreased surgical trauma and lower incidence of postoperative complications [2] and is used in protocols aiming to enhance surgical recovery and simultaneously reduce complications in the field of thoracic surgery (Enhanced Recovery After Surgery – ERAS) [3]. From an anesthetist’s (and patients’) point of view less invasive techniques are connected with additional benefits such as a smaller surgical incision, decreased pain intensity in the postoperative period, and, last but not least, the possibility to reduce the requirement for intraoperative use of strong opioids. This can be achieved by introducing anesthesia techniques aimed at reducing adverse events and complications associated with strong opioids in large doses. One approach is using different types of regional anesthesia. Another way is using opioid-free anesthesia (OFA). Our aim in this study was to compare two regimens of perioperative pain management – OFA coupled with paravertebral block (PVB) versus standard opioid balanced general anesthesia with simple multimodal analgesia.
Opioid-free anesthesia
OFA is an anesthetic management technique that was initially designed for the purpose of bariatric surgery, mainly to achieve a reduction in opioid consumption in a group of patients extremely prone to complications connected with their use – obese individuals [4] – and has since grown, becoming more popular in different surgical specialties such as general surgery (it has been used successfully in laparoscopic cholecystectomy [5], spinal surgery [6] and even cardiac surgery [7]). The main idea behind this anesthetic management strategy is that no opioid drugs are used for induction and maintenance of an anesthetic – this includes intravenous, neuraxial and tissue infiltration use [8]. The homeostasis of the patient in the intraoperative period is achieved by using a combination of different groups of medications including nonopioid analgesics and adjunct drugs, such as propofol, dexmedetomidine, lidocaine, magnesium, and ketamine, to produce anesthesia, sympatholysis, and analgesia [6].
Thoracic paravertebral block (ThPVB)
PVB usage is well established as an effective regional anesthesia technique in thoracic surgery [9]. It has been proven in many studies to be a safe and reliable alternative to thoracic epidural anesthesia (TEA) [10] and a useful adjunct in multimodal pain management after VATS [11]. It is also recognized as a standard treatment in pain management after VATS by the PROSPECT working group [11]. It is relatively easy to perform and has a favorable risk to benefit ratio. Using this regional anesthesia technique makes it possible to achieve good analgesia and further reduce the intraoperative requirement for opioids.
As the surgical and anesthesia techniques rapidly evolve, we wanted to find out whether it is feasible to apply the principles of OFA coupled with PVB to achieve an effective and safe anesthetic for VATS wedge pulmonary resection and to establish whether the benefits are extended to the immediate postoperative period (first 24 hours).
METHODS
This is a sub-analysis focusing on OFA feasibility and postoperative pain management of a previously published, randomized controlled study, conducted at a single university hospital (SPSK no. 1 im. Prof. S. Szyszko 3 Maja 13-15 41-800 Zabrze, Poland) of the Medical University of Silesia, Poland [12].
After gaining the approval of the Institutional Review Board (No KNW/0022/KB1/41/16) and obtaining written informed consent from the subjects, we enrolled 66 patients scheduled for elective VATS pulmonary wedge resection. The study was registered at ClinicalTrials.gov as No. NCT04355468, completed according to the Declaration of Helsinki and compliant with Good Clinical Practice.
The time frame of the study was between December 2015 and March 2018. We wanted to make sure that surgery type and tissue trauma would be comparable between the groups. Because the video-thoracoscopic approach to pulmonary lobectomy was becoming more popular in our facility, meaning a decreasing number of VATS pulmonary wedge resections, it took more time than anticipated to screen and enroll a sufficient number of potential study participants. The second reason why the study took so long was because it was our intention for all general and regional anesthetic procedures to be performed by a small number of physician anesthetists to limit interpersonal variability.
Study inclusion criteria
All enrolled patients were aged between 18 and 65, had a body mass index (BMI) within 19–30 kg m–2, and an American Society of Anesthesiology (ASA) physical status between I and III.
Study exclusion criteria
Exclusion criteria were: lack of consent, significant coagulopathy, contraindication to ThPVB or drugs used in protocol, a history of chronic pain, chest wall neoplastic invasion, previous thoracic spine surgery, thoracic trauma or previous thoracic surgery, mental state preventing effective use of intravenous patient-controlled analgesia (PCA) device, renal failure (glomerular filtration rate (GFR) < 60 mL min–1 1.73 m–2) or any type of hepatic failure, as well as pregnancy, lactation and substance abuse. Patients with morbid obesity (BMI > 30) were also excluded from the study, because of technical difficulties with performing PVB, which was considered pivotal for anesthetic management in the study group.
Study interventions
Patients were randomly assigned to receive either standard general anesthesia with subsequent pain management based on intravenous PCA delivered opioid – oxycodone (CG – control group), or preope-rative PVB combined with OFA and subsequent pain management based on intravenous PCA delivered opioid – oxycodone (OFA group). Random assignment was ensured by using a sequence generat-ed by www.randomizer.org. Allocation concealment was ensured as the numbers were put into sealed opaque envelopes and randomly chosen by the anesthetists scheduled to administer anesthesia. All anesthetic interventions were performed by 3 physician anesthetists (two attending-level physicians and one senior anesthesia resident) with at least 4 years of experience and an adequate number of previously performed PVBs (over 50).
Opioid-free anesthesia group (OFA)
In the OFA group a preoperative single-shot PVB was performed at the Th3 to Th4 level, approximately 2.5 to 3 cm lateral to the tip of the spinous process. An ultrasound examination was undertaken prior to performing the block to assess the depth of the transverse process and the pleura. An insulated 10 cm long needle was used, and this was connected to a peripheral nerve stimulator with an initial set current of 2.5 mA. The current was gradually reduced as the needle was inserted until the appearance of visible intercostal muscle activity with a current of 0.3 to 0.5 mA (paravertebral space identification). After assuring that the tip of the needle was located in the paravertebral space, plain bupivacaine (0.3 ml kg–1) was injected after a negative aspiration test for air or blood. Loss of sensation to cold was checked after 20 min with a plastic ampoule of saline kept in a freezer. Testing was symmetrical on both sides of the thorax. A difference in the cold sensation between the blocked and unblocked sides was chosen as the end point to identify a successful block. After PVB was performed and induction to general anesthesia was completed, a continuous intravenous infusion of lidocaine and ketamine was started according to the predefined dosing regimen:
1) Bolus dose of lidocaine (Lignocainum Hydrochloricum WZF, Polfa Warszawa S.A., Poland) 1.5 mg kg–1 i.v. followed by a continuous infusion at a dose of 2.0 mg kg–1 h–1 for 2 hours, then reduced to 1.2 mg kg–1 h–1 and maintained throughout the study period, meaning 24 hours postoperatively.
2) Bolus dose of ketamine (Ketalar, Pfizer, Poland) 0.35 mg kg–1 i.v. followed by a continuous infusion at a dose of 0.2 mg kg–1 h–1 for 2 hours, then reduced to 0.12 mg kg–1 h–1 and maintained throughout the study period, meaning 24 hours postoperatively.
3) Bolus dose of esmolol – a short acting beta blocker (80 mg), followed by an infusion if necessary (150 μg kg–1 min–1) was administered in case of a rise in blood pressure or heart rate (HR) over 20% above the baseline value.
Control group (CG)
In CG patients surgical analgesia was achieved by using a strong opioid drug – fentanyl (FENTANYL WZF, Polfa Warszawa S.A., Poland). A dose of 1.5 µg kg–1 was used for anesthesia induction, and subsequently, fractional doses of fentanyl 1 to 3 µg kg–1 were administered if the patient’s HR or mean blood pressure (MBP) rose more than 20% above the baseline value obtained just before surgery commenced, which was predefined as a sign of experiencing pain.
In both groups, general anesthesia was induced with midazolam 0.1 mg kg–1, propofol 2 mg kg–1, and cisatracurium 0.15 mg kg–1. Patients were intubated using a left-sided double-lumen tube (DLT) of an adequate size. After confirming the proper position of the DLT, the patient was positioned in a late-ral decubitus position and surgery commenced. Anesthesia was maintained with one minimal alveolar concentration (1 MAC) of sevoflurane. Patients were awaked from anesthesia in the post-anesthesia care unit (PACU), where extubation was performed by a physician anesthetist after administration of incremental doses of atropine and neostigmine, as required.
The postoperative pain management schedule was identical in both groups. During the stay in the PACU, if a patient complained of pain, then she/he was given intravenous oxycodone by an anesthetist before starting the patient on the i.v. PCA device. This dose was titrated to achieve adequate analgesia or until side effects occurred. The opioid used in the PCA solution was oxycodone (1 mg mL–1), and the device was programmed to allow a self-administered bolus dose of 1 mg of oxycodone with a lockout time of 5 min. Additionally, patients were given 1 g of intravenous paracetamol every 6 h and 100 mg of intravenous ketoprofen every 12 h.
Study outcomes
Demographic data (age, sex, height, weight, BMI) and vital information from previous medical history such as present comorbidities were recorded during pre-op anesthetic assessment. Baseline hemodynamic parameters including heart rate (HR; bpm), non-invasive blood pressure (NIBP; mmHg) – systolic blood pressure (SBP; mmHg), diastolic blood pressure (DBP; mmHg) and mean blood pressure (MBP; mmHg) – were also noted at this time.
During anesthesia, all patients were monitored by 3-lead electrocardiography (ECG), end-expiratory carbon dioxide (EtCO2– mmHg) and sevoflurane (EtSev MAC value and vol%) concentration and arterial blood saturation measured by pulse oximetry (SpO2). HR (bpm), NIBP (mmHg), SBP, DBP, and MBP were also recorded.
In the immediate postoperative period and the first 24 hours after surgery, the following parame-ters were assessed at predefined time points: HR (bpm), SpO2, NIBP (mmHg), and respiratory rate (RR). We also tracked the level of sedation with the Ramsay score, the pain intensity level with the Prince Henry Hospital Pain Score (PHHPS), which is particularly useful in pain assessment after thoracic surgical procedures as it takes into consideration the presence or absence of pain on coughing and breathing deeply, and pain levels with the Numerical Rating Score (NRS). The details of the scale are presented in Table 1. Pain intensity assessment was performed every 4 hours.
TABLE 1
Prince Henry Hospital Pain Score (PHHPS)
| PHHPS pain assessment scale | |
|---|---|
| 0 | No pain on coughing |
| 1 | Pain on coughing but not on deep breathing |
| 2 | Pain on deep breathing but not at rest |
| 3 | Slight pain at rest |
| 4 | Severe pain at rest |
Oxycodone consumption within 24 hours and pain intensity on the NRS at rest were selected as the primary outcomes. Postoperative nausea and vomiting (PONV), the need for additional analgesics, adverse events of methods and drugs used in study protocol (with special focus on opioid-related respiratory complications), and additional medical interventions related to pain were also recorded.
Statistical analysis
Data with normal distribution and documented on an interval scale are presented as mean (standard deviation – SD). Data without normal distributions and ordinal data are presented as median with upper, lower quartiles, minimum, and maximum. Qualitative data are presented as n.
The Shapiro-Wilk test was used for evaluation of the normal distribution of the presented data. For comparison between the groups, Student’s t-test was used for independent variables (homogeneity of variances was tested with Levene’s test) and the Mann-Whitney U test for other data.
To compare dichotomous variables, we used the χ2 test with Yates’ correction where necessary. For the variability of the parameters over time and their differences between the groups, we used parametric variance analysis with repetitive measurements and post hoc contrast analysis. A recursive, weighted least squares estimation method was used for fitting a regression model of the variability of studied data overtime. A P-value lower than 0.05 was considered statistically significant. P-values were corrected with Bonferroni correction for multiple comparisons.
RESULTS
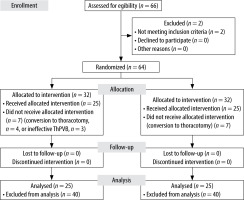
During the study period, 66 patients scheduled for elective VATS pulmonary wedge resection were screened for eligibility as study subjects. In total, 64 patients fulfilled the inclusion and exclusion criteria and were randomly assigned to the two study groups (32 patients in each group). The participant flow diagram is presented in Figure 1. Overall, 14 patients were excluded after randomization: 7 patients from the OFA group (4 had a conversion to thoracotomy; 3 had ineffective PVB, which was connected with inability to perform OFA in accordance with the predefined regimen and would generate risk in a form of delivering inadequate analgesia; it was not possible to redo the block without exceeding the maximal dose of bupivacaine) and 7 patients from the control group (conversion to thoracotomy). Finally, 50 patients (21 males and 29 females) with a median age of 59 ± 5 years and a BMI of 27 ± 2 kg m–2 completed the study. Demographics and main clinical findings are presented in Table 2.
TABLE 2
Patient demographic characteristics
There were no significant differences between studied groups in terms of age, gender, BMI, or ASA physical status. Except for MBP, there was no significant difference between the groups in their baseline hemodynamic parameters and this diffe-rence was not considered to be clinically relevant as the size effect was rather small. Also, no significant differences were found between the groups regarding the incidence of chronic diseases (Table 3), except for occurrence of lung cancer, which was more pre-valent in the OFA group.
TABLE 3
Comparison of comorbidities in the studied groups
| Group | Yes | No | P-value | |
|---|---|---|---|---|
| HA | Control | 16 | 9 | 0.387 |
| OFA | 14 | 11 | ||
| CAD | Control | 7 | 18 | 0.500 |
| OFA | 8 | 17 | ||
| DM | Control | 1 | 24 | 0.174 |
| OFA | 4 | 21 | ||
| LC | Control | 20 | 5 | 0.025 |
| OFA | 25 | 0 | ||
| ND | Control | 5 | 20 | 0.371 |
| OFA | 7 | 18 | ||
| COPD | Control | 2 | 23 | 0.209 |
| OFA | 5 | 20 | ||
| NDS | Control | 1 | 24 | 0.305 |
| OFA | 3 | 22 | ||
Within 24 hours of postoperative observation, hemodynamic parameters, such as HR, SBP, DBP, and MAP, did not show statistically significant differences between the groups. There was no need for use of intraoperative esmolol in both study groups.
The level of oxygenation remained stable in all study subjects; the only significant difference was noted at one predefined time point, 16 hours after the surgery – SpO2 was significantly higher in the control group. Seemingly contradictory to previous findings, the respiratory rate was significantly higher in the OFA group at any time observed.
Overall the pain levels experienced by patients in both groups were acceptable. The pain level measured on the PHHPS scale was significantly different between the groups only 20 hours and 24 hours after surgery (respectively; P = 0.015, P = 0.021) in favor of the OFA group (Table 4). The differences in NRS values between groups were not significant, being only of borderline significance at the 24 h point of observation (P = 0.056; Table 5).
TABLE 4
Comparison of PHHPS in the studied groups
TABLE 5
Comparison of NRS in the studied groups
A statistically significant difference in postoperative oxycodone consumption was observed (P = 0.035). The average 24-hour oxycodone consumption was 11.2 mg (SD 5.4) in the OFA group and 18.0 mg (SD 4.4) in the control group. Comparing the demand for opioids between the groups on the first day after surgery, significantly higher values were found in the CG (P < 0.001; Table 6). This is in line with the fact that patients in the CG required rescue analgesia and additional interventions more often, although these differences were not significant (respectively: P = 0.284; P = 0.269; Table 7). By additional interventions we refer to the procedures that needed to be performed during patient care and connected with pain management such as administration of rescue drugs and more frequent monitoring and follow-up visits.
TABLE 6
Comparison of oxycodone consumption [mg]
| Group | Mean | SD | Min. | Qu1 | Me | Qu3 | Max. | P-value |
|---|---|---|---|---|---|---|---|---|
| Control | 18.0 | 4.4 | 12.0 | 15.0 | 18.0 | 21.0 | 26.0 | < 0.0001 |
| OFA | 11.2 | 5.4 | 4.0 | 6.0 | 11.0 | 15.0 | 20.0 |
TABLE 7
Comparison of PONV, rescue analgesia, additional interventions and oxygen therapy in the studied groups
| Group | Yes | No | P-value | |
|---|---|---|---|---|
| PONV | Control | 1 | 24 | 0.095 |
| OFA | 5 | 20 | ||
| Rescue analgesia | Control | 12 | 13 | 0.284 |
| OFA | 9 | 16 | ||
| Additional interventions | Control | 19 | 6 | 0.269 |
| OFA | 16 | 9 | ||
| Oxygen therapy | Control | 11 | 14 | 0.128 |
| OFA | 16 | 9 |
Sedation level measured on the Ramsay scale was similar between groups throughout the whole time of observation (P > 0.05). No serious adverse events were recorded in both groups. There was one episode of agitation requiring pharmacological treatment in the OFA group recorded as an adverse event. There was a greater number of PONV episodes in the OFA group, but this result was not significant (P = 0.095).
DISCUSSION
Although the use of opioid drugs has been a state-of-the-art practice in anesthesia and postoperative pain management after major surgery, there is emerging evidence that it may not only be associated with dangers in the immediate intra- and postoperative period (e.g. respiratory depression, bradycardia, hypotension, PONV) but also affect long-term outcomes, e.g. potential development of opioid addiction. Many addicts unfortunately start their addiction during a routine surgical admission to hospital [13]. This is one of the reasons why techniques aimed at reducing the amount of opioid drugs that the patient consumes throughout their hospital stay are receiving more attention [14].
OFA has been advocated for various procedures such as surgery for the morbidly obese, chronic opioid addicts, patients with sleep apnea, and cancer surgery [15].
OFA’s main benefits in breast surgery and gynecology are better pain management in the postoperative period, nausea, and vomiting prevalence reduction, as well as decreased inflammation of surgical wounds. The authors strongly recommend that patients with sleep apnea syndrome undergoing “one-day” surgery, as well as patients suffering from opioid intolerance or immune disorders, should be anesthetized with OFA [16].
Other benefits are also proposed. Frauenknecht et al. [16] prepared a systematic review and meta-analysis of randomized clinical trials (RCTs) evaluating opioid-inclusive versus opioid-free anesthetic regimens comparing immediate postoperative pain and PONV. Based on data analyzed from 23 RCTs, they concluded that use of intra-operative opioids did not influence postoperative rest pain at 2 hours after the surgery and was associated with an increased incidence of PONV. More recent data were presented by Feenstra et al. [17], with similar findings.
However promising those findings are, every new treatment concept that comes into the spotlight should be carefully evaluated and approached with due skepticism. Likewise, OFA is not without its flaws.
The first problem is the lack of a clear definition of what exactly the term “OFA” means. This creates a methodological flaw resulting from the heterogeneity of the studies and makes the conclusions of the aforementioned meta-analysis less reliable [15].
The second issue is the lack of defined and validated methods to ensure that we are indeed delivering adequate care, in terms of effective surgical analgesia. Evidence for reliable objective pain monitoring during OFA is limited [17].
Last but not least, using OFA is not associated with any significant difference in opioid usage when considering the intraoperative period, postoperative opioid usage, and the amount of opioid drugs the patient is prescribed when discharged from the hospital [15, 17]. Recently a definition of a reduction in opioid usage throughout the entire intra- and postoperative hospital stay was proposed, also tackling the concept of OFA. This concept was defined as a “peri-operative care strategy that maximizes non-opioid modalities for anaesthesia and analgesia and reserves the use of opioids for severe acute pain not relieved by other methods from admission to discharge from the hospital” [15]. This concept of opioid stewardship, or, in other words, opioid sparing perioperative management, is a way to increase the patient safety and contribute to further improvement in anesthesia practice overall. We strongly agree with this statement.
As there is a need to develop procedure-specific regimens for OFA [18], thoracic surgery will probably remain one of the fields with the greatest challenges in achieving reliable analgesia during surgery and in the postoperative period.
Pain after thoracic surgery is severe and can cause several complications such as impairing respi-ration, which contributes to pulmonary atelectasis [19]. Increased oxygen demand with coronary artery disease may cause ischemic events. Reduced mobility and inability to undergo rehabilitation increase the risk of thromboembolic complications. Elderly patients may experience mental disorders, often with episodes of depression, anxiety, or agitation [20]. All abovementioned features make our intended population quite challenging.
The presented data are a sub-analysis of a population that has already been studied before by our group. The results were published in a previous paper that focused on objective pain assessment during surgery using a skin conductance algesimeter [12].
In the current study we wanted to demonstrate that it is feasible to perform anesthesia for VATS pulmonary wedge resection with restricted use of opioid drugs, by using a combination of regional anes-thesia (thoracic PVB), non-opioid analgesics, and adjunct drugs leading to reduction of the amount of opioid drugs necessary for adequate pain control 24 hours after surgery. The CG received a standard anesthesia regimen with similar postoperative pain management. Pain levels were considered acceptable in both groups and the amount of postoperative oxycodone consumption was significantly lower in the OFA group.
All patients were initially qualified for VATS pulmonary wedge resection, but 11 were converted to thoracotomy. In 3 patients from the OFA group, PVB was ineffective, which prevented them from being anesthetized with the OFA regimen. This finding proves that this type of anesthesia is not completely reliable but is consistent with other authors who reported similar incidence of failed blocks [20]. According to other authors, an anesthesiologist’s experience significantly increases the chance of correct location of the paravertebral space and thus affects the quality of anesthesia. It is possible that the goal of the most effective location of the paravertebral space would be to use a peripheral nerve stimulator [21] or performing the block under real-time ultrasound guidance. The number of complications that can occur in a relationship with thoracic PVB varies between 1.8 and 10%. The most dangerous are inadvertent spinal anesthesia, pneumothorax, pulmonary hemorrhage, and serious neurological complications [22, 23]. In our study, the percentage of failed blocks was 9.37%, with no other serious complications.
Many scientific studies regarding adverse effects of using opioid drugs in the postoperative period have been published over the years. The most severe and widely known complications include respiratory depression, which is commonly seen in elderly patients and those with sleep apnea syndrome [24, 25]. As already mentioned above, the level of oxygenation remained stable in all study subjects; the only significant difference was noted at one predefined time point – SpO2 was significantly higher in the CG, but the size of the effect is clinically negligible as none of the patients experienced a marked decrease in SpO2. Of interest, and seemingly counterintuitive in light of previous findings, RR was significantly higher in the OFA group at any time observed. In our opinion this could be attributed to better pain management during movement with PVB, but as the time frame exceeds the time of effective block, this conclusion is still to be confirmed. No episodes of respiratory depression were observed in either group. This can be attributed to the relatively low dose of opioids needed for adequate pain control due to co-administration of non-opioid analgesics. Also the chosen drug administration mode, via a PCA intravenous pump, provided effective control, preventing the patient from taking high doses of an opioid in a short period of time.
Patients were also evaluated for sedation levels on the Ramsay scale. Both excessive sleepiness and productive symptoms such as hallucinations, delirium, involuntary movements, and general psychomotor agitation may occur after general anesthesia [26, 27]. In this study, only one episode of agitation with the need for pharmacological treatment was noted.
In both groups, PONV episodes were observed, but more events occurred in the OFA group. In the literature, higher prevalence of PONV has been reported in patients who received higher doses of opioids [28], which contrasts with our observation in the present study, but the difference was small and did not reach statistical significance. One of the possible causes is the use of neostigmine together with atropine – we did not find a statistically significant difference between the study group and the control group in terms of its use, but this topic deserves further research.
Another important topic to consider is the pre-valence of chronic postoperative pain, which is a significant problem after thoracic surgery. Preventing chronic pain after thoracic surgery involves a multifaceted approach, including effective perioperative pain management strategies. Employing regional anesthesia techniques, such as thoracic PVB, can provide targeted pain relief and mitigate the risk of chronic pain development. Also ketamine was studied for this purpose with success [29].
CONCLUSIONS
Proper pain control in the postoperative period is essential not only for the patient’s comfort but also for the possibility of rehabilitation and quick recovery. The pain management proposed in our study for the first 24 h after VATS pulmonary wedge resection in both groups was satisfactory. Both types of anesthesia were safe and reliable. There were no respiratory complications directly related to the use of the study drugs and no significant difference in hemodynamic parameters. The use of OFA allowed the reduction of opioid use in the postope-rative period. Moreover, regional anesthesia, such as PVB as a supplement to general anesthesia, is part of the multimodal anesthesia approach and this combination offers further benefits.
Therefore, OFA combined with ThPVB as a part of “opioid sparing” anesthetic management can be a feasible alternative in this type of surgery, especially in patients with contraindications to opioid use.
LIMITATIONS
There are some limitations of our study. First of all, the sample size was small, and the results should be confirmed in a larger study. No statistically significant difference was found between the studied group and the control regarding the majority of investigated outcomes, but in some cases (e.g. pain assessment values in NRS at some of the predefined time points) there were non-significant differences which might have been significant if the groups had been larger. Another limitation is restricting the time of observation to 24 hours. We decided on this time frame because of the anticipated time of an effective PVB, but it would also be interesting to know if there is any difference in measured variables when the time of observation is prolonged. It should also be emphasized that in our study group there were 3 block failures, which makes this technique not completely reliable. As it was explained before, some groups of patients benefit from OFA more than others. Unfortunately there was a small percentage of patients suffering from chronic obstructive pulmonary disease (COPD) in our study participants, and we did not screen them for obstructive sleep apnea (OSA), which could have resulted in missing vital information. Also restricting the BMI value of the patients due to anticipated technical difficulties in performing PVB could be seen as a limitation.