Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, and by 2030, it is predicted to be the primary cause of liver transplantation. Its rising prevalence is associated with increased metabolic syndrome, obesity, and insulin resistance [1, 2]. NAFLD presents a vast clinical spectrum from simple steatosis without liver fibrosis to cirrhosis caused by non-alcoholic steatohepatitis (NASH) [3]. NAFLD patients have a higher risk of adverse events compared to the general population. However, mortality risk is not the same across the spectrum, with NASH-related cirrhosis posing the greatest risk of mortality [4-6]. Early identification of a cohort with liver fibrosis is of paramount importance so that early counseling and treatment, as well as a referral to a transplantation center, can be performed. Because of the critical nature of early identification, an ongoing effort to identify other risk factors that may take part in the development of fibrosis is essential.
Thyroid hormone is essential for lipid and glucose metabolism, as well as mitochondrial function in the liver [7]. Hypothyroidism, both overt and subclinical, has been demonstrated to be an independent risk factor for the development of NAFLD [8]. However, its association with liver fibrosis, the more significant clinical entity, is less clear. We aimed to summarize the association between hypothyroidism and liver fibrosis risk.
Material and methods
Study selection
Our meta-analysis adhered to PRISMA guidelines. Two independent investigators conducted a literature search of PubMed and ProQuest from inception to June 30, 2021, restricting the search to studies involving humans and articles published in English. We used medical subject headings and free-text terms for the keywords, as shown in Table 1.
Table 1
Search strategy
Eligibility criteria
We included studies if they met the following criteria: 1) the study was a cohort, case-control, or cross-sectional study, 2) the study estimated the association between the presence of hypothyroidism and risk of liver fibrosis or NASH, 3) the study reported odds ratios (ORs) with their respective 95% confidence intervals (CIs) or presented raw data. When the study carried out analyses with adjustment for confounding factors, we chose the adjusted over unadjusted OR. The excluded papers were: 1) studies with causes of liver fibrosis other than NAFLD spectrum, 2) short communication, correspondence, letters to the editor, conference abstracts, review, or case reports, 3) desirable data could not be retrieved, 4) full-text articles were not available, 5) non-English articles.
Assessment of bias risk
The risk of bias of included studies was separately assessed by two investigators using the Newcastle-Ottawa Scale (NOS). Studies with an NOS score < 7 were considered to have a high risk of bias, whereas those with a score ≥ 7 were considered to have a low risk of bias.
Data extraction
The same two investigators independently extracted the following data from each study using a prespecified form: author, year, study design, country, sample size, hypothyroidism definition, NAFLD diagnosis criteria, NASH, significant liver fibrosis, advanced liver fibrosis diagnosis criteria, and adjustment used. We contacted authors for additional data or clarification when needed.
Statistical analysis
We estimated the impact of hypothyroidism on the risk of liver fibrosis and NASH through pooled OR with their corresponding 95% CI. Data were pooled based on fixed-effects or random-effects assumptions. P-values < 0.05 were considered to be significant. Higgins’ I2 statistic was used to assess heterogeneity. If the value of I2 was < 50%, the fixed-effect models could be applied. Otherwise, random effects could be used. We planned to assess publication bias through a funnel plot if included studies reached the minimum number of 10. An asymmetric plot could suggest publication bias. All statistical analyses were performed using Review Manager version 5.4 (The Cochrane Collaboration).
Results
After deleting duplicates and reviewing titles and abstracts for eligibility, 203 of the 216 potential records identified by the search approach were excluded. Eight studies were found to be eligible for qualitative and quantitative synthesis after additional full-text inspections for 13 articles (Fig. 1).
Study characteristics
The baseline characteristics of the eight studies are summarized in Table 2. The studies involved 14,588 patients, ranging from 103 to 9,419 patients in each study, originating from 7 different countries. Studies were published between 2003 and 2021. Four studies reported the association between overt hypothyroidism and NASH risk. Four studies investigated the impact of subclinical hypothyroidism, defined as an increased level of serum thyroid stimulating hormone (TSH) with normal FT4, and two studies, by Kim et al. [9] and Martínez-Escudé et al. [10], used a TSH cut-off of ≥ 2.5, also known as low-normal thyroid function. Low-normal thyroid function was said to be more robust than subclinical hypothyroidism in detecting cardio-metabolic consequences [11, 12]. The diagnosis criteria of NASH, significant liver fibrosis, and advanced liver fibrosis varied across studies, as shown in Table 2.
Table 2
Study characteristics
[i] BMI – body mass index, BP – blood pressure, FIB-4 – Fibrosis-4 index, FLI – fatty liver index, FT4 – free T4 hormone, GGT – γ-glutamyl transferase, HDL – high density lipoprotein, HOMA-IR – homeostatic model assessment for insulin, HTN – hypertension, IU – international unit, kPa – kilopascal, LS – liver stiffness, NAFLD – non-alcoholic fatty liver disease, NA – not applicable, TG – triglyceride, TSH – thyroid stimulating hormone, US – ultrasonography, WC – waist circumference
Quality of included studies
The study quality ranged from 6 to 8, as shown in Table 3. Four studies were found to have a high risk of bias, whereas four were found to have a low risk of bias.
Publication bias
We were not able to conduct publication bias assessment through the funnel plot as the included studies in the subgroup analysis did not reach 10 studies.
Subclinical hypothyroidism and significant liver fibrosis
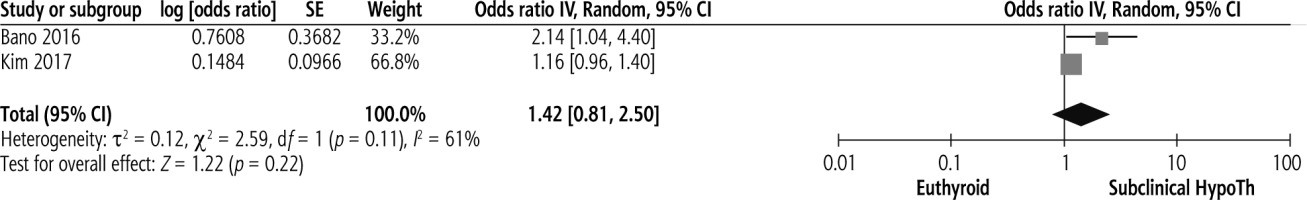
Studies investigating the association between subclinical hypothyroidism and significant liver fibrosis are limited. Pooled results on the presence of subclinical hypothyroidism and the risk of liver fibrosis are shown in Figure 2. Presence of subclinical hypothyroidism was not significantly associated with significant liver fibrosis (pooled OR = 1.42, 95% CI = 0.81-2.50, p = 0.22).
Subclinical hypothyroidism and advanced liver fibrosis
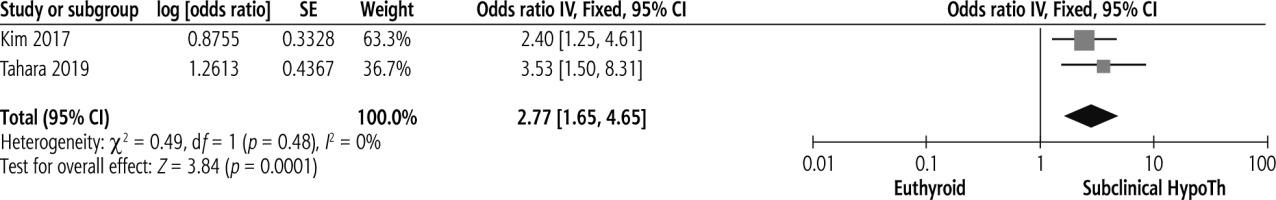
Subclinical hypothyroidism was significantly associated with advanced liver fibrosis, with pooled OR of 2.77, 95% CI = 1.65-4.65, p < 0.00. No heterogeneity was present, as shown in Figure 3 (I2 = 0%, p = 0.48).
TSH ≥ 2.5 and significant liver fibrosis
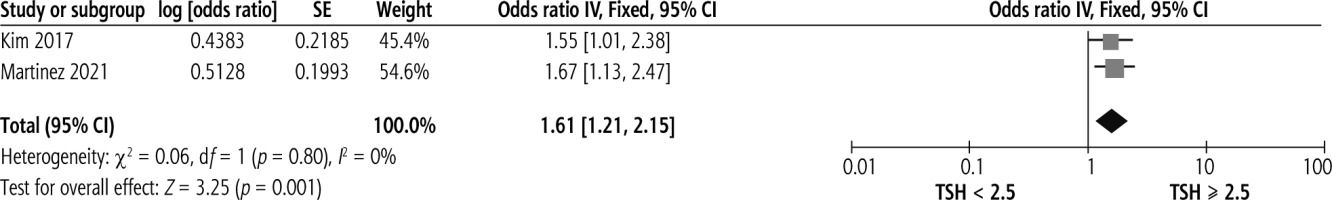
Two studies investigated the association between a TSH cut-off of ≥ 2.5 and significant liver fibrosis in NAFLD patients. A significant association was found between TSH ≥ 2.5 and significant liver fibrosis, shown in Figure 4, with no heterogeneity (OR = 1.61, 95% CI = 1.21-2.15, I2 = 0%).
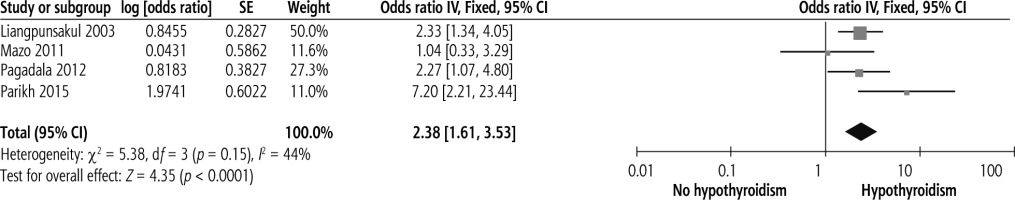
Hypothyroidism and NASH
Four studies assessed the relationship between overt hypothyroidism and NASH risk. Overt hypothyroidism was significantly associated with NASH risk with no heterogeneity, as shown in Figure 5 (OR = 2.38, 95% CI = 1.61-3.53, I2 = 44%).
Discussion
Interpretation
The current research summarized the association between hypothyroidism and liver fibrosis risk from 8 observational studies. From our pooled results, subclinical hypothyroidism was significantly correlated with the development of advanced liver fibrosis in NAFLD patients. There was also a significant association between TSH cut-off of 2.5 and significant liver fibrosis. A significant association between overt hypothyroidism and NASH risk was also reported. However, no significant relationship was found between subclinical hypothyroidism and significant liver fibrosis.
The underlying mechanism has not been fully elucidated. Prior studies have demonstrated various mechanisms underlying the association between hypothyroidism, both subclinical and overt, with NAFLD. Thyroid hormone regulates major metabolic processes, one of which is intrahepatic lipolysis, mediated by lipophagy. Thyroid hormone also promotes lipid droplet entrapment, destruction of the lipid, and accelerated fatty acid oxidation, which will result in reduced steatosis in the liver [13]. In hypothyroid state, a decline of hepatic lipase activity triggered by reduced triglyceride clearance and hepatic triglyceride deposition can induce NAFLD. De novo lipogenesis, as well as a movement of free fatty acids to the liver, can also occur due to insulin resistance associated with hypothyroidism [14-16]. Reports from animal studies have stated that the use of an agonist agent targeting the thyroid receptor in the liver has been shown to reduce liver steatosis [17, 18]. Evidence regarding its use in humans, although limited, has been documented. Levothyroxine supplementation was proven to show benefit in a randomized clinical trial of 363 subclinical hypothyroid patients with dyslipidemia by significantly reducing the steatosis in the liver [19].
However, its association with liver fibrosis, the more important clinical entity, is less clear. Pathophysiologically speaking, the underlying mechanism may be due to the accumulation of extracellular matrix [20]. Prior research revealed that thyroid hormone might play a regulatory role in the activation of stellate cells in the liver, thus also suggesting its involvement in liver fibrosis signaling [21-24]. Experimental studies on myocardium have also indicated that hypothyroidism can induce upregulation of collagen type 1 gene expression, causing subsequent fibrosis [25-27]. Administration of thyroid hormone promotes metalloproteinase activity, leading to more collagen degradation [28]. This suggests a potential effect of thyroid hormone as NAFLD and fibrosis treatment. Some studies have started to report drugs under development targeting thyroid hormone receptors in the liver as potential nonalcoholic steatohepatitis (NASH) treatment [29, 30]. Another mechanism may also be explained by hepatocellular damage induced by thyroid dysfunction-related oxidative stress. A low thyroid hormone level may disrupt existing adipokines in circulation, such as tumor necrosis alpha, adiponectin, and leptin, causing subsequent hepatic inflammation and liver fibrosis [16, 31, 32]. Additionally, autoimmune-related hypothyroidism is also hypothesized to mediate the association between hypothyroidism and liver fibrosis. Several investigations have revealed that patients with Hashimoto’s thyroiditis were more likely to have fibrosis compared to euthyroid [33, 34].
Our meta-analysis presented a significant association between hypothyroidism and liver fibrosis risk despite variation of diagnostic methods. A novel cut-off of TSH ≥ 2.5, also known as low-normal thyroid function, has been rigorously studied previously, as it better reflects various health problems compared to the traditional cut-off, such as insulin resistance, prediabetes, dyslipidemia, atherosclerotic disease, chronic kidney disease, worse outcome in heart failure patients, and other common cardiometabolic disorders in several population studies [12, 35-37]. However, to date, only three studies have investigated the association between low-normal thyroid function and NAFLD, as well as liver fibrosis risk [9, 10, 38].
Strengths and limitations
This is an updated meta-analysis of the association between hypothyroidism and the risk of liver fibrosis in NAFLD patients. Although having a similar result to a prior meta-analysis [39], our study also presents summarized novel evidence regarding the cut-off of thyroid function which better reflects cardiometabolic disorders. Low-normal thyroid function is reported to be associated with a modest increase of plasma total cholesterol, LDL cholesterol and triglycerides, thus conveying a pro-atherogenic process which contributes to advanced oxidative stress, which is a huge contributor to liver fibrosis in NAFLD patients. Thus, screening, which then leads to the early treatment of subclinical hypothyroidism, might be of clinical benefit in reducing the risk of liver fibrosis in NAFLD patients [11, 12].
However, our meta-analysis has some limitations. First, the number of powered studies was limited. Second, the methods of NAFLD and fibrosis diagnosis varied and could have contributed to the bias. Significant liver fibrosis in included studies was assessed variously through different methods, through liver stiffness measurement using transient elastography with a cut-off of ≥ 8 kPa and liver biopsy according to Brunt and Kleiner criteria. Advanced liver fibrosis was also estimated by a surrogate marker (FIB-4 index) in 1 study and liver biopsy in another one. Third, because the nature of the studies were all observational, potential cause and effect relationships could not be determined for certain. These data still need to be confirmed with further prospective interventional studies, incorporating thyroid hormone replacement.