Boerhaave syndrome is a rare condition characterized by a spontaneous rupture of the esophagus, typically caused by forceful vomiting [1]. It is associated with a high mortality rate, reaching up to 30% [2]. The first recorded description of an esophageal emetic lesion dates back to 1724 and is credited to the Dutch physician and philosopher Hermann Boerhaave. Symptoms initially manifest in a nonspecific manner, with clinical findings commonly including chest pain, vomiting, and subcutaneous emphysema, collectively known as Mackler’s Triad [1]. The primary etiology of Boerhaave syndrome is barogenic, primarily resulting from severe and prolonged vomiting, as well as activities such as defecation and weightlifting [3]. Early detection within 24 h is crucial to reduce the mortality rate significantly, from 40% to 6.2% [3]. However, the diagnosis is often complicated by the presence of various other differential diagnoses, including perforated gastric or duodenal ulcers, acute myocardial infarction, pericarditis, pneumothorax, pulmonary thromboembolism, diaphragmatic hernia, dissolving aortic aneurysm, and acute pancreatitis. Treatment should encompass both surgical intervention and broad-spectrum antibiotics as patients are at a high risk of rapid sepsis development. The management approach for esophageal perforation should be determined based on the size of the defect.
A 32-year-old male was admitted to the Intensive Care Unit (ICU) at his local hospital due to acute respiratory distress syndrome (ARDS) and diabetic ketoacidosis (DKA). The patient had a significant medical history of uncontrolled diabetes, esophageal candidiasis, pancreatitis, and alcohol and drug addictions. What is more, 2 days before admission, the patient experienced hematemesis and diarrhea. Laboratory tests revealed mild anemia, elevated C-reactive protein (CRP) and potassium levels, and significantly high glucose levels. Physical examination indicated epigastric tenderness and pain, desaturation, and increased heart and respiratory rates. Due to decreased respiratory sounds and suspected edema, pleural drainage was performed, which revealed stomach content mixed with blood. A total of 5 l of fluid were obtained from both pleural cavities during the hospitalization. To decompress the stomach, a nasogastric tube was inserted. A computed tomography (CT) scan with oral contrast showed fluid effusion from the gastrointestinal tract into the right pleural cavity, along with partial atelectasis of the lower lobe of the left lung and signs of fluid reactive reaction. The patient was sedated, intubated, and mechanically ventilated with synchronized intermittent mandatory ventilation. A packed red blood cell (PRBC) transfusion was administered. Total parenteral nutrition was initiated, necessitating the placement of a central venous catheter. Exploratory laparoscopy was performed, revealing no abdominal pathologies.
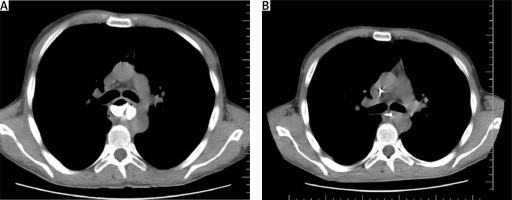
Following the diagnosis of esophageal perforation, the patient was transferred to the Thoracic Surgery Department in our Center. An esophageal repair was performed utilizing omental pedicle flap transplantation through a right-sided thoracotomy. A 5 cm long rupture was visualized in the cardiac orifice area, approximately 40 cm from the dental line. In addition, gastrostomy and jejunostomy procedures were conducted via laparotomy. Postoperative computed tomography with oral contrast did not detect esophageal leakage, pneumothorax, or fluid accumulation. The lungs were properly expanded with effective drainage. After 1 month, the patient developed stenosis below the sutured perforation site, prompting the decision to place a self-expanding esophageal stent (Figure 1).
Figure 1
A – Contrast detection above the stenosis; B – esophageal stenosis below the perforation suturing
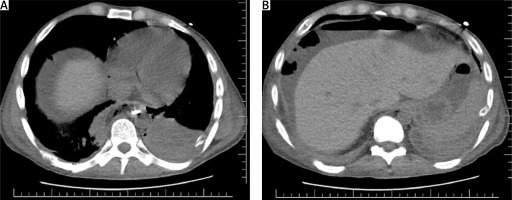
At the 3-month follow-up, an endoscopy revealed the presence of an esophageal fistula located 25 cm from the dental line. Esophagobronchial fistulas are relatively uncommon, occurring in approximately 4% of cases. However, the risk of developing such fistulas increases with longer periods of stent placement and the use of longer stents. The proximal and middle segments of the esophagus are more commonly affected [4]. In our patient, pre-existing conditions such as esophageal candidiasis and diabetes may have contributed to the increased risk of developing a fistula associated with the presence of an esophageal stent. As a result, the patient underwent laparotomy, during which the esophageal stent was removed. Then, under gastroscopic guidance, endoluminal vacuum therapy (EVT) was administered. A polyurethane foam was wrapped around the tip of a nasogastric tube and applied to the esophageal fistula. EVT therapy was initiated with a negative pressure of –100 mm Hg. The sponges were changed every 3–4 days, and EVT was discontinued after 28 days. The patient’s nutrition was solely administered via a percutaneous endoscopic gastrostomy-jejunostomy (PEG-J) tube throughout this period. Follow-up chest CT confirmed complete healing of the esophageal fistula and revealed contrast passing through the stenosis, indicating resolution of the narrowing in the esophagus. We observed significant clinical improvement and alleviation of the patient’s symptoms (Figure 2).
Physicians must maintain a high level of suspicion for Boerhaave syndrome in patients presenting with severe vomiting and chest pain, as early diagnosis and treatment are vital in reducing mortality. While other more common conditions like myocardial infarction, pulmonary embolism, and pancreatitis may be initially considered in the differential diagnosis, missing a diagnosis of Boerhaave syndrome can have substantial risks of death. In this case, the diagnosis of Boerhaave syndrome was based on the classic Mackler’s triad of symptoms, including severe vomiting, chest pain, and subcutaneous emphysema. Imaging modalities such as upright chest X-rays and CT scans played a crucial role in confirming the diagnosis by detecting abnormalities like pneumomediastinum, pleural effusion, and contrast extravasation. Multiple factors could have contributed to or exacerbated the esophageal rupture in this case. Severe vomiting, leading to increased intragastric pressure and lack of coordination between the upper and lower esophageal sphincter, resulted in transmural rupture of the esophageal wall. Additionally, severe esophageal inflammation caused by Candida albicans infection may have affected the muscular layer and contributed to the rupture [5]. The localization of the rupture in the distal third of the esophagus is a distinguishing feature from Mallory-Weis syndrome. While there may be some overlap in clinical features between the two syndromes, such as increased intraluminal esophageal pressure, there are significant differences that aid in making the final diagnosis. Mallory-Weis syndrome typically occurs in the gastric cardia due to repeated retching causing lacerations. Unlike Boerhaave syndrome, Mallory-Weis syndrome is characterized by mild pain and massive hematemesis, whereas Boerhaave syndrome presents with severe pain and mild hematemesis. In the emergency department, the immediate priority is to stabilize the patient through fluid resuscitation, administration of antibiotics, analgesia, and nasogastric decompression. Urgent surgical consultation should be obtained as most esophageal perforations require repair, either through primary closure or reinforcement with tissue flaps. Non-operative management with aggressive medical therapy may be considered for contained leaks detected very early.
Esophageal perforation is a life-threatening condition, with a mortality rate of up to 30% if not treated within 24 h [6]. Managing this condition can be challenging due to nonspecific presentations, such as chest pain, radiation of pain to the neck and arm, and exacerbation of symptoms with breathing. Non-specific symptoms like chest pain, vomiting, pain radiating to the arm, and worsening with breathing can further complicate the clinical presentation. Although some cases may be managed conservatively with broad-spectrum antibiotics, most perforations require surgical repair. Endoscopic vacuum therapy is a novel approach that should be considered as a standard of care in surgical patients [7]. In the emergency medicine setting, clinicians should provide supportive therapy including antibiotics, acid suppression, nasogastric suction, and transitioning patients to parenteral feeding.