PURPOSE
Multiple sclerosis (MS) and systemic lupus erythematosus (SLE) are autoimmune diseases whose incidence is steadily increasing. MS affects about 2.9 million people worldwide and 51,000 in Poland, while SLE affects 3.4 million people worldwide and 18,500 in Poland [1, 2]. MS is a chronic, demyelinating disease of the central nervous system (CNS), one phenotype of which is the primary progressive form (PPMS). This occurs in about 10-15% of MS patients. The course of this form of the disease is characterized by a constant progression of neurological damage without periods of exacerbation or remission [3]. SLE manifests mainly through the pathology of the musculoskeletal system, joints, skin, and kidneys. However, 39-50% of patients with SLE have nervous system involvement under the name of neuropsychiatric lupus (NPSLE), which can manifest as demyelinating disorders in 0.9-2.7% of cases [4]. However, the coexistence of these diseases in a single patient is extremely rare (18 cases recorded worldwide according to Jácome Sánchez et al., 2018) [5]. Therefore, the appropriate differential diagnosis of MS and NPSLE and the selection of treatment represent a specific challenge for the neurologist. The purpose of the following paper is to describe these challenges via a case report of the coexistence of PPMS and SLE in a single patient.
CASE DESCRIPTION
A 54-year-old woman with suspected PPMS since 2022 was admitted to the Department of Neurology in April 2023 due to the worsening of her neurological condition. Previously, in 2013, the patient was diagnosed with SLE. Laboratory tests revealed the presence of antinuclear antibodies (ANA) at a titer of 1 : 320 (positive result ≥ 1 : 80), anti-double stranded DNA (anti-dsDNA) antibodies at a concentration of 48 IU/ml (positive result > 45 IU/ml) and 171.4 U WHO/ml (positive result > 139 U WHO/ml). Additionally, the presence of lupus anticoagulant antibodies (LAC) was detected (no information on concentration). The patient had been treated with hydroxychloroquine and methotrexate. She remained in remission from her rheumatic disease.
On admission to the Clinic, the patient reported weakness in her extremities, with greater severity in the lower extremities, deterioration of her gait, impaired balance and superficial sensation in the lower extremities. The aforementioned symptoms had persisted for two weeks, with a marked increase for two days. On neurological examination the patient was conscious, in logical verbal contact, and meningeal symptoms were negative. Nystagmoid movements of the left eyeball when looking to the left, features of central damage of the right facial nerve, quadriparesis more severe in the right and lower extremities (3/5 on the Lovett scale), and weakness of superficial sense of touch and pain in the right extremities were found. In addition, Babinski’s sign was present on the right side. The Romberg test was unstable, and gait inefficient with small steps on a wide base (5/10 on the Tinetti scale). The results of the laboratory tests performed, including inflammatory markers, remained within the range of reference norms (C-reactive protein [CRP] – 0.705 mg/l, leukocytes level – 8.58 x 103/μl). A computed tomography (CT) scan of the head was performed to exclude vascular pathology of the brain – no abnormalities were shown. Steroid treatment with methylprednisolone was administered, with no improvement in neurological status. Diagnosis of PPMS was established according to McDonald’s criteria: worsening disability for at least one year, typical brain MS-like lesion (Figure I), typical lesions in the spinal cord (Figure II), and positive test for oligoclonal bands in the CSF. The presented MRI brain lesions met the diagnostic criteria for MS according to Barkhof and Tintoré (Table 1). After a rheumatology consultation and the exclusion of contraindications in laboratory tests, the decision was made to give the patient 600 mg of ocrelizumab after premedication. No early complications were observed. The administration of ocrelizumab resulted in a good clinical response – currently (October 2023) the patient can move using a walker. She is waiting for the next injection of the preparation.
Figure I
A) Magnetic resonance imaging (MRI) scan of the brain in T2-weighted image in FLAIR sequence, (B) axial plane, (C) MRI scans of the brain in T2-weighted image, sagittal plane. Multiple sclerosis lesions according to Barkhof and Tintore MRI criteria for primary progressive multiple sclerosis [14]
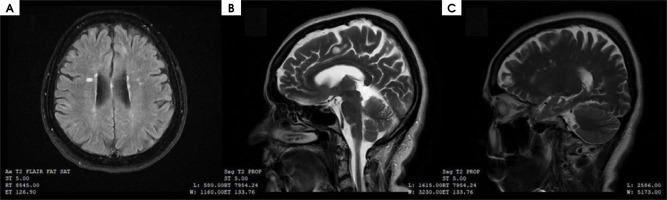
Figure II
Magnetic resonance imaging scan of the spinal cord in T2-weighted image, sagittal plane. Multiple sclerosis lesions in the spinal cord (hyperintense areas)
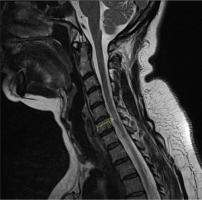
Table 1
Diagnostic criteria for multiple sclerosis in magnetic resonance imaging scans according to Barkhof and Tintore [14]
COMMENT
Autoimmune diseases are characterized by a continual increase in incidence, as well as frequent co-occurrences of conditions involving various organs and systems. One such disease is MS. It can coexist with various autoimmune diseases – most notably autoimmune thyroiditis, type 1 diabetes mellitus, psoriasis or inflammatory bowel diseases [6]. There are also reports of MS co-occurring with Sjögren’s syndrome, rheumatoid arthritis, Behçet’s syndrome or systemic scleroderma [7]. The coexistence of these diseases is due to a genetic susceptibility to autoimmunity and their related pathomechanisms. Interestingly, the co-occurrence of MS and another autoimmune disease does not cause a clinical exacerbation of either of them. Fanouriakis et al., in a study on 9 patients with MS and SLE, showed that both diseases were mild, and that SLE remained in remission during MS treatment [8]. It was proved that MS has a milder course if it co-occurs with inflammatory bowel diseases but the coexistence of MS and psoriasis, type 1 diabetes mellitus and autoimmune thyroiditis exacerbated the process of demyelination and neurodegeneration in the radiological images of the patient’s brain [9]. The rarity of the co-occurrence of MS and SLE is justified primarily by the activity of different immune signaling pathways in these two diseases. In MS, upregulation of transforming growth factor β (TGF-β) and tumor necrosis factor α (TNF-α) signaling pathways is observed, while impaired activation of these pathways is evident in SLE, with an increase in the signal transducer and activator of transcription 4 (STAT4) axis. Common mechanisms include activation of STAT1, STAT3, interferon γ (IFN-γ) pathways and dysregulation of STAT5 phosphorylation [10]. Due to the small number of reports, it is difficult to outline the epidemiology of the co-occurrence of MS and SLE. However, MS and SLE have been shown to occur more frequently in members of one family and monozygotic twins [11]. Coexistence in one person in a study of 9 patients showed that this was more often the case for women with an average age of 42, and that the diagnosis of the diseases was an average of 5 years away (SLE came first in 55% of cases). This applies to the relapsing-remission type of MS [8]. In our literature review we found only three cases involving PPMS, one of which showed symptoms similar to the one we described [8,11]. Only one case report concerned a patient who experienced sensory-motor disorders. However, she was much younger than our patient (21 vs. 54 years old). The other two reports concerned patients with spinal manifestations of MS, also in younger people (34 and 18 years old). The antibody profile in all of them remained consistent with that in our patient (ANA [+] and ds-DNA [+]). All patients were treated with hydroxychloroquine and azathioprine [11]. The differences between the patients described may result primarily from the advancement and activity of rheumatic disease – all of them had SLE in the active phase, with arthritis, thrombocytopenia, fever or skin symptoms. Our patient’s disease had previously been mild, without the involvement of the kidneys or joints, and at the time of her stay at the Department of Neurology she was in remission. Undoubtedly, the SLE condition affects both the symptoms and the outcome of MS treatment in the patient. Curiously enough, the occurrence of drug-induced lupus erythematosus (DIL) was also found in MS patients treated with IFN β-1b, natalizumab and alemtuzumab. No such reaction was observed after the administration of glatiramer acetate [7]. This is due to the fact that inflammation in SLE is exacerbated, and mediated, by IFN [7]. In the treatment of co-existing MS and SLE, in addition to the use of corticosteroids, rituximab appears to be a suitable drug with proven efficacy in both conditions, but detailed studies and management guidelines are lacking [5]. Furthermore, the use of IFN for therapeutic purposes significantly improves the condition of MS patients but causes severe exacerbation in SLE, especially in lupus nephritis. For this reason, it is important to differentiate between the two diseases and be able to apply appropriate, disease-specific treatment. The most important features to consider in the differential diagnosis of the two diseases are shown in Table 2. Nikolopoulos et al. noted the relevance of CSF and MRI studies in differentiating between MS and SLE. In the CSF of patients with NPSLE, IgG index > 0.65 (25% vs. 86%, p = 0.002) and positive oligoclonal bands (25% vs. 79%, p = 0.006) were observed far less frequently. In MRI images, spinal cord, infratentorial (p < 0.05), periventricular (p < 0.01), and juxtacortical (p < 0.05) areas were significantly less frequently involved in patients with NPSLE than in MS patients. Involvement of the optic nerve occurred with similar frequency in both groups. In addition, three out of four SLE patients had only one brain area affected, whereas spatial dispersal is characteristic of MS [12]. It should also be remembered that combining drugs that affect the immune system, especially biological drugs, should be carefully considered, and the patient’s condition after administration and any side effects should be monitored.
Table 2
Differences between multiple sclerosis (MS) and nervous system involvement in the course of systemic lupus erythematosus (SLE) [5, 13]
MS and SLE are two autoimmune diseases that can, extremely rarely, coexist in a single patient. Therefore, it is necessary to describe cases of such co-occurrence so that the epidemiology and appropriate management of patients can be determined. This will also help to understand the incompletely known pathogenesis of both diseases.







