Introduction
Approximately 10–20% of colorectal cancer (CRC) patients have a family history of the same disease [1], and 5–7% of CRCs have a clear genetic origin [2]. Hereditary CRC syndromes are divided into CRC with polyposis and CRC without polyposis (i.e. Lynch syndrome) [3]. Risk factors for CRCs include inflammatory bowel disease (i.e. Chron’s and ulcerative colitis) [4].
The chronic inflammation (i.e. Chron’s and ulcerative colitis) is associated with release of inflammatory cytokines with subsequent chronic DNA and cell damage [3]. The chronic DNA/cell damage and/or genetic mutations predispose to the development of premalignant polyps or lesions [5]. The premalignant polyp can be further differentiated into either adenoma-carcinoma (70–90%) or serrated CRC (10–20%). The non-polyposis CRC had lower incidence than the polyposis type [3].
The microbiome includes microorganisms found in the host’s epithelial and/or mucus membrane barriers, acquired since birth by vertical transmission, modulated throughout the life of the host by the environment, and reacting with the host in a complex way [6, 7].
The microbiome maintains the health of the epithelial and/or mucus membrane barriers, involved in the development of diseases and it is modulated by the host’s immune system [8]. The gut microbiota metabolites, short-chain fatty acids (SCFAs) [9] (i.e. acetate and butyrate) [10] produced by the bifidobacteria have a protective effect against CRC [11]. SCFAs reduce the inflammatory cytokines [11], inhibit colonocyte proliferation, and promote malignant cell apoptosis [12].
Butyrate maintains the integrity of the mucus membrane barrier, regulates the occlusion of the mucosal cell junction, and reduces intestinal mucosal inflammation [13].
Butyrate-producing bacteria such as genus Butyricicoccus, can protect against colitis in irritable bowel syndrome [14]. SCFA-producing bacteria are essential for healthier microbiota profiles [15].
Reduced butyric acid level and increased inflammatory cytokines were observed after reduced Bacteroides fragilis and Bacteroides vulgatus species in the colon [16]. Akkermansia muciniphila bacterium decreased in patients with ulcerative colitis [17] and in those with CRC [18].
Dysbiosis means a pathological change and/or imbalance of intestinal microbiota composition, which is associated with tumour development [19, 20]. Intestinal microbiota imbalance (decreased beneficial microbiota and increased pathogenic organisms) is frequently observed in CRC patients, caused by intensive use of antibiotics [21].
Chronic inflammation and disruption of the intestinal mucosal are the most important risks for CRC [22]. H2S-producing bacteria are associated with CRC development. H2S-producing bacteria can oxidize and reduce local intestinal SCFAs [23].
H2S is toxic to colonocytes, it oxidizes the butyrate, damages intestinal mucosal epithelium, and promotes chronic inflammation with a subsequent DNA damage [24]. Additionally, H2S disrupts the balance between cell proliferation and apoptosis [25]. High H2S concentrations were observed during stool examination of 100 patients with CRCs [26]. Other studies reported increased Fusobacterium nucleatum species (H2S-producing species) in colonic tumour tissue [27, 28].
The bacterial species associated with development of CRCs include B. fragilis, E. coli (Escherichia coli), Peptostreptococcus spp., Streptococcus bovis, and Enterococcus faecalis [29, 30]. B. fragilis and E. coli produces local onco-toxins (i.e. B. fragilis toxin and colibactin) [31], which increased the tumour growth and mortality from CRCs in animal studies [31].
Although the value of nutrition is frequently underestimated, the available data show that nutrition is crucial in shaping the gut microbiota. The type of food is the main way to interact and modify gut microbiota [32]. Nutritional diseases such as malnutrition and obesity produce major alternations in gut microbiota. A plant-based food can change our microbiota faster than other therapeutic measures. The Mediterranean diet increases the beneficial gut microbiota and the SCFAs [33]. The high-fibre diet decreases the risk of CRCs through increased gastrointestinal motility and decreased contact of pro-cancerous metabolites/toxins to the gut mucosal barrier, and production of SCFAs when fermented by the gut bacteria [34].
Probiotics are “living bacteria that have a beneficial health effect when given in suitable amounts” [35]. The benefits of probiotics range from the maintenance of intestinal mucosal barrier integrity to the prevention of irritable bowel disease [36]. Probiotics modify the gut microbiota, reduce inflammatory cytokines, and secrete anti-cancer metabolites [37]. Additionally, probiotics modulate T-lymphocyte and dendritic cell activities [38]. In vitro [39] and clinical studies [40–42] reported beneficial health effects of probiotics. Akkermansia muciniphila (A. muciniphila), is one of the probiotics that has a beneficial health effect on obesity [43] and/or epithelial tumours [44].
Moreover, lactic acid-producing bacteria have been used for their immunomodulatory effect [45] and Lactobacillus salivary and Lactobacillus fermemtum combined with Lactobacillus acidophilus reduced CRC cell proliferation in experimental studies [46, 47].
Prebiotics are defined as “substances utilized by the host’s organism that have a beneficial health effect” [48]. Inulin is an example of the prebiotics and dietary fibres (i.e. inulin-type fructans) found in garlic, onion, and asparagus [49]. Inulin-type fructans are not digested in the small intestine and are fermented in the colon to produce lactic acid and SCFAs [50, 51] from the CRC protective bacteria (i.e. Bifidobacteria [52], Bacteroides [53], and A. muciniphila) [43].
Resistant starch is another example of prebiotic (not digested in the small intestine and fermented in the colon), found in cereals, legumes, vegetables, and seeds. Resistant starch produces protective SCFAs (butyrate) and reduces the colonic PH [54].
Symbiotics are “a mixture of living bacteria (probiotics) and substances utilized by the host’s organism (prebiotics), and both have a beneficial health effect” [55].
An example of a symbiotic associated with decreased incidence of CRC includes a combination of Bifidobacterium animalis subspecies lactis (B. lactis) as a probiotic and resistant starch (prebiotic) [55].
The role of microbiota has been studied with different cancer treatments, including radiotherapy [56], chemotherapy [57], and immunotherapy [58]. Most studies have focused on the microbiota’s role in immunotherapy because of its promising role in immune response modulation [58].
Paulos et al. [56] reported the microbiota’s role in immune response modulation. Paulos et al. [56] found that the Gram-negative bacteria lipopolysaccharide (LPS) increases the activity of dendritic cells and TCD8+ lymphocytes, with subsequent melanoma regression after radiotherapy.
Immunotherapy is still ineffective in a large proportion of CRCs, due to the host’s genetics and/or cancer phenotype [59]. Immunotherapy induces the host’s immune response through T-lymphocytes. Therefore, it is important to identify the ideal microbiota to improve the efficacy of immunotherapy during CRC treatment. The beneficial microbiota during immunotherapy treatments for epithelial tumours include Clostridiales, Ruminococcacae, Enterococci, Collinsella, Alistipes, A. muciniphila, B. fragilis, and Bifidobacteria [60].
The aim of surgeries in CRC is total tumour resection with safety margins around. The risk of tumour recurrence significantly decreases if the CRC is totally resected with tumour-free margins around [61].
However, the incidence of local recurrence in CRCs is 15%, which is due to the histological and biological characteristics of the primary tumour and/or the degree of deep invasion [62, 63]. Mostly the tumour recurrence occurs at the anastomosis site due to loss of the intestinal mucosal barrier’s integrity and the presence of collagenolytic organisms [64]. A high-fat diet increases the recurrence of CRCs at the anastomosis site in preclinical studies [65] due to overproduction of collagenolytic organisms at the anastomosis site [65]. Therefore, low collagenolytic organisms after CRCs surgeries is a healthy colonic microbiota for successful CRC surgeries and prevention of recurrence.
Although, the value of nutrition is frequently underestimated in medical fields, the available data showed that nutrition is crucial in shaping the gut microbiota.
Aim
This systematic review aimed to detect the effect of microbiota modulation using probiotics, prebiotics, symbiotics, and natural changes on CRCs.
Material and methods
Inclusion criteria: A PubMed search was conducted to retrieve the original and in vivo articles published in English language from 2010 until 2021 containing the following keywords: 1) CRCs, 2) CRCs treatment (i.e. surgical, chemotherapy, radiotherapy, and/or immunotherapy), and 3) microbiota probiotic(s), prebiotic(s), symbiotic(s), dysbiosis, and/or nutritional treatment.
Exclusion criteria: in-vitro studies, commentaries, letters to the editor or correspondence, editorials comments, duplicate publications, retractions, and reviews (narrative, systematic, and/or meta-analyses) were excluded.
Results
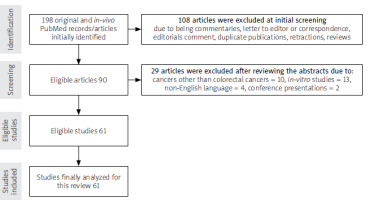
A total of 198 PubMed records/articles were initially identified. 108 articles were excluded at the initial screening because of the article type (i.e. commentaries, letters to the editor or correspondence, editorials comment, duplicate publications, retractions, reviews). After reviewing the abstracts, another 29 articles were excluded due to cancers other than CRCs, in vitro studies, non-English language, and conferences presentations. Finally, 61 studies were eligible and were analysed for this systematic review (Figure 1).
Table I contains a summary of the reviewed studies including authors and publication year, cancer origin, type of the study, intervention, effect of intervention on the microbiota and tumour, and possible mechanisms that explain the effect of intervention.
Table I
Summary of the reviewed studies
| Authors and publication year | Origin of cancer | Type of the study | Intervention | Effect of intervention on microbiota | Effect of intervention on tumour | Possible mechanism that explains the effect of intervention |
|---|---|---|---|---|---|---|
| Preclinical probiotics studies: | ||||||
| Shi et al. 2020 [75] | CRC model | Mice injected subcutaneously with CT26 cells (colorectal carcinoma cell line) ± IL-2 | A. muciniphila | Increased Akkermansia, Allstipes, and Lactobacillus (L.) | Decreased tumour volume and increased survival rate | Increased tumour necrosis and apoptosis and decreased tumour proliferation. Increased interferon-γ and IL-2 in the tumour and increased interferon-α in serum |
| Arthur et al. 2013 [71] | CRC model associated with colitis | 129/SvEv mouse strain deficient in IL-10 treated with AOM | VSL#3 (contains, L. plantarum, L. delbrueckii, L. bulgaricus, L. paracasei, L. acidophilus, B. breve, B. longum, B. infantis and Streptococcus salivarius) | Increased mucosal proteobacteria. Decreased Bacteroidetes in feces | Increased tumour penetration, multiplicity, and histological grade | Decreased clostridium in the mucous membrane |
| Zhuo et al. 2019 [38] | CRC with colitis | Male BALB/C mice strain treated with AOM and DSS | L. acidophilus lysates (Phylum firmicutes) | Decreased Proteobacteria | Decreased tumour volume and increased survival rate | Increased lymphocytes (CD3+ CD4+), interferon-γ and macrophages in mesenteric lymphoid |
| Chandel et al. 2019 [94] | Early colon carcinogenesis | Male rats treated with DHM | L. rhamnosus and L. plantarum | Increased lactic acid producing-bacteria | Decreased aberrant intestinal crypts | Decreased weight loss, and liver transaminases |
| Song et al. 2018 [67] | CRC with colitis | C57BL/6 mice strain treated with AOM and DSS | E. faecalis, B. longum and L. acidophilus | Increased Lactobacillus. Decreased Desulfovibrio, and Mucispirillum | Decreased number and volume of tumours | Decreased IL-1β, IL-6 and TNF-α |
| Wang et al. 2020 [18] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | A. muciniphila or its membrane protein (Amuc_1100) | Increased A. muciniphila | Decreased malignancy score | Increased cytotoxic CD8+ lymphocytes and TNF-α in mesenteric nodules |
| Xu et al. 2021 [113] | CRC associated with colitis | 6-C57BL/6NCrSlc mice strain treated with AOM and DSS | L. rhamnosus (M9) | Increased Akkermansia, L. and Bifidobacterium | Decreased number, size of the tumour, and inflammatory markers | Decreased CD68+ and CD163+ |
| Chang et al. 2020 [114] | CRC associated with colitis | CRC in animal model treated with DHM | Butyricicoccus pullicaecorum | Butyricicoccus pullicaecorum | Decreased tumour infiltration, bleeding, and carcinoembryonic antigen (CEA) levels | Increased SLC5A8 and GPR43 |
| Hradicka et al. 2020 [115] | Early colon carcinogenesis | CRC in rates treated with DHM | L. plantarum (VD23, C28 and MS18). L. salivarius (MS3, MS6 and MS16) | NR | Decreased proliferation, size, and number of tumours | Increased IL-18 |
| Silveira et al. 2020 [95] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | L. bulgaricus | L. bulgaricus | Decreased tumour mass and size | Decreased IL-6, IL-17, IL-23 and TNF-α. Increased interferon-γ in healthy and tumour tissue |
| Yuan et al. 2018 [66] | Syngeneic CRC model | BALB/c mice strain injected subcutaneously with CT26 cells (colorectal carcinoma cell line) | L. and Bifidobacterium | Increased Alloprebotella, Citrobacter , Roseburia, Thalassospira, Erysipelatoclostridium, Bacteroides_chinchillae Helicobacter_ Ganmani. Decreased Escherichia coli, and Bacteroides vulgatus | No change in tumour volume | NR |
| Chang et al. 2018 [72] | Syngeneic CRC model | BALB/c mice strain injected subcutaneously with CT26 cells (colorectal carcinoma cell line) and treated with FOLFOX | L. casei variety rhamnosus (Lcr35) | Increased Firmicutes and Bacteroidetes | Unchanged | Decreased diarrhea, mucous membrane inflammation, TNF-α and IL-6 |
| Bindels et al. 2018 [116] | CRC model with cachexia | Mice injected subcutaneously with CT26 cells (colorectal carcinoma cell line) | Faecalibacterium prausnitzii | NR | Decreased tumour volume (insignificant) | Improvement parameters of the intestinal barrier (insignificant) |
| Jacouton et al. 2017 [73] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | Lactobacillus casei BL23 | Increased Lactobacillus zeae | Decreased tumour volume, and number | Decreased Ki67 |
| Gao et al. 2017 [74] | CRC associated with colitis | BALB/c mice strain treated with AOM and DSS | Lactobacillus reuteri | NR | Decreased tumour numbers | Decreased tumour IL-22, IL-6, IL-1-α and TNF-α |
| Chung et al. 2017 [70] | CRC associated with colitis | BALB/c mice strain treated with AOM and DSS with Western diet and metformin | VSL#3 | NR | Metformin + VSL#3 decreased number of tumours | Metformin + VSL#3 decreased Ki67 |
| da Silva Duarte et al. 2020 [117] | Early colon carcinogenesis | BALB/c mice strain treated with DHM | L. paracasei L. rhamnosus | Increased Ruminiclostridium and fecal acetic acid and SCFAs | Unchanged | Decreased IL-6. Increased TNF-α, interferon-γ and the P-53 (tumour suppressor gene) |
| Mendes et al. 2018 [69] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | L. acidophilus, L. rhamnosus and Bifidobacterium bifidum | Increased L., B., Allobaculum, and Clostridium | Decreased number of tumours | Decreased colitis, and TNF-α. Increased IL-10 |
| Preclinical prebiotics studies: | ||||||
| Donohoe et al. 2014 [34] | CRC model with colitis | BALB/C mice strain treated with AOM and DSS | Fructooligosac charide/inulin | NR | Decreased histological grade, volume number | Increased histone 3 and decreased Ki-67 |
| Zhang et al. 2021 [81] | Syngeneic model of colon cancer | C57BL/6 WT mice strain injected with colon cancer (MC38) cells, treated with humanized microbiota from healthy humans or patients, anti-PD-1 treatment | Pectin | Increased Lactobacillaceae, Bifidobacteriaceae, Ruminococcaceae, and Faecalibacterium. Increased acetate and butyrate | Decreased tumour volume and size | Increased lymphocytes CD4+, TCD8+ and interferon-γ |
| Li et al. 2020 [83] | Subcutaneously transplanted colon cancer cells (MC-38) | Syngeneic C57BL/6 mice strain | Mucin and inulin | Increased Clostridium cluster XIVa | Inulin decreased tumour volume. Tumour volume unchanged with mucin | Increased CD40+ and TLR3 and TLR7 |
| Zhu et al. 2021 [118] | CRC with colitis | C57BL/6 mice strain treated with HFD, AOM and DSS | Evodiamine | Increased Campylobacter, B., and L. Decreased E. faecalis and E. coli | Increased survival and apoptosis. Decreased tumour diameter and proliferation | Decreased IL-1, IL-6 and circulating TNF-α and in colonic tissue |
| Terasaki et al. 2021 [100] | CRC associated with colitis | ICR mice strain treated with AOM and DSS | Fucoxanthin | Increased Lachnospiraceae and decreased Bacteroides and Rikenellaceae | Decreased size and number of colorectal adenomas | Increased caspase 3 |
| Luo et al. 2021 [119] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | Menthol | Increased butyrate producing bacteria | Decreased the number of 1– 3 mm adenomas in a dose-dependent manner | Decreased expression of Ki67, IL-6 and TNF-α. Increased IL-10 in the distal colon |
| Liu et al. 2020 [120] | Subcutaneous transplanted colon cancer (MC-38) | Mice | Chitosan and LMW citrus pectin encapsulating bilberry anthocyanins | Increased Lachnospiraceae (Clostridiales) and diversity | Decreased tumour volume | Increased of SCFAs and T lymphocytes (CD4+ and TCD8+) |
| Zhang et al. 2019 [89] | CRC associated with colitis | C57BL/6 mice strain treated with AOM, DSS and HFD | Canmei formula | Increased Bacteroides, Bacteroidaceae, Faecalibaculum, Erysipelatoclostridium and Staphylococcus | Decreased tumour incidence | Decreased serum IL-17 |
| Khan et al. 2019 [92] | Prevention of CRC of genetic origin | Mice | Polysaccharides and saponin | Increased SCFAs producing bacteria | Decreased number and size of polyp | Shift from M1 to M2 macrophages. Reversal of Ecadherin/N-cadherin ratio and decreased oncogenic signaling |
| Li et al. 2020 [83] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | α-ketoglutarate | Increased Akkermansia, Butyricicoccus, Clostridium and Ruminocococcus. Decreased Escherichia and Enterococcus | Decreased tumour size and stage | Decreased IL-1, IL-6, IL-22, and TNF |
| Fernandez et al. 2018 [121] | CRC associated with colitis | Rats treated with AOM and DSS | Lactulose derived Galacto-oligosaccharides | Increased B., Bacteroidaceae, Prevotellaceae, Acidaminococcaceae, Bifidobacteriaceae, and Peptococcaceae. Decreased Lachnospiraceae, Eubacteriaceae, Acholeplasmataceae | Decreased number and volume of polyps | Increased cecum length |
| Mudd et al. 2020 [122] | CRC associated with dysbiosis | Mice inoculated with Bacteroides fragilis | Anthocyanidins | NR | Decreased number of tumours | Increased liver phase I enzymes CYP1A1 and CYP1B1 |
| Wu et al. 2018 [87] | CRC associated with colitis | Males ICR mice strain treated with benzo[a]pyrene and DSS | Polymethoxyflavones | Increased Sphingobacteriaceae, Gammaproteobacteria, and Ruminococcaceae. Decreased Bacilli, Parabacteroides, L. ruminis | Decreased hyperplasia, adenoma, and adenocarcinoma | Decreased hepatic and renal toxicity. Decreased Inflammation and metastasis genes. Increased antioxidants |
| Wang et al. 2019 [15] | CRC associated with colitis | FVB/N mice strain treated with benzo[a]pyren e and DSS | Epigallocatech in gallate | Increased L., Fusobacterium, Ruminocococcus. Decreased Bacteroides | Decreased volume and number of tumours and pre-cancerous lesions | NR |
| Chou et al. 2017 [90] | CRC associated with colitis | ICR mice strain treated with AOM and DSS | Boswellia serrata resin extract | Increased Clostridiales spp. and decreased Bacteroidales | Decreased number of tumours | Decreased TNF-κB. Increased colon length |
| Fukuda et al. 2011 [123] | CRC associated with colitis | AOM-treated rats | Germinated barley extract | NR | Decreased aberrant crypts | Decreased TLR4 and COX2 |
| Preclinical symbiotics studies: | ||||||
| Zheng et al. 2020 [91] | Subcutaneous and orthotropic model of CRC | CRC model in mice | Clostridium butyricum and prebiotic dextran | Increased Muribaculaceae, Bacteroides, Mucispirillum, Alloprebotella, Lachnospiraceae, and Ruminococcaceae | Decreased tumour size in combination with Diclofenac | Increased iso-butyrate, butyrate, and isovalerate, propionate |
| Oh et al. 2020 [96] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | Lactobacillus gasseri 505 and leaf extract of Cudrania tricuspidata (CT) in fermented milk | Increased Lactobacillus, Bifidobacterium, and Akkermansia associated with SCFAs | Decreased incidence of tumours and dysplasia | Decreased TNF-α, interferon-γ, IL-1β, IL-6 |
| Preclinical food studies: | ||||||
| Gaines et al. 2020 [65] | Model of CRC recurrence after surgery | BALB/c mice strain inoculated with CT26 cells (colorectal carcinoma cell line) after resection and anastomosis | Western diet, antibiotics, and Enterococcus faecalis | Western diet associated with increased proteus, Akkermansia and Trabulsiella. Decreased in Bacteroides, Roseburia and Ruminococcus | Increased distant metastasis with Western diet | NR |
| Rudd et al. 2019 [98] | CRC genotoxic | C57BL/6 mice strain treated with AOM | Selfish Muricidae | NR | 6-Br decreased the number of aberrant crypts and tumours. NE decreased number of tumours | 6-Br decreased tumour proliferation. NE decreased proliferation and increased apoptosis |
| Zhang et al. 2021 [81] | CRC associated with colitis | BALB/c mice strain treated with AOM and DSS | Extract of 55 different foodstuffs | Decreased Desulfovibrio and Ruminococcaceae. Increased Bacteroides, Ruminiclostridium_6 and Allobaculum. Increased acetic, ascorbic, palmitic, and branched amino acids | Decreased number and size of tumours. Increased survival rate | Improved intestinal barrier and body weight. Decreased IL-6 and TNF-α |
| Piazzi et al. 2019 [104] | CRC genotoxic | CRC in mice model treated with AOM | LFD HFD A mixture of one of them with MD | MD improves dysbiosis with an HFD | MD decreased incidence of CRCs | MD increased apoptosis especially in LFD. MD increased EPA, especially in LFD |
| Li et al. 2015 [101] | Prevention of CRCs of genetic origin | CRCs in mice model | Nutmeg | NR | Decreased tumour volume and number of tumours | Decreased cresol sulphate and phenyl sulphate in urine. Decreased serum IL-6, liver transaminases and amylase |
| Chen et al. 2018 [93] | CRC associated with colitis | C57BL/6 mice strain treated with AOM and DSS | Anthocyanin extract from blackberries | Decreased Eubacterium rectale, Faecalibacterium prausnitzii Lactobacillus, Desulfovibrio and Enterococcus spp. | Decreased number of tumours | Decreased IL-1β, IL-6, IL-10 and TNF-α |
| Fernandez et al. 2018 [121] | CRC associated with colitis | Rats treated with AOM and DSS | Anthocyanin extracted from strawberries and blackberries | Decreased Bilophila wadsworthia | Decreased number of tumours | Improved plasma reducing capacity |
| Wang et al. 2019 [15] | CRC associated with colitis | Mice treated with AOM, DSS and HFD | American Ginseng | Improving dysbiosis | Decreased number of tumours and histological grades | NR |
| Hu et al. 2016 [88] | CRC associated with colitis | Rats treated with AOM and DSS | Green tea extract (EGCG) and resistant starch | Increased Parabacteroides, Barnesiella, Ruminococcus, Marvinbryantia and Bifidobacterium. Increased acetate, and butyrate | Resistant starch decreased number of adenocarcinomas | Decreased TNF-α and IL-1β |
| Yu et al. 2015 [124] | Prevention CRCs of genetic origin with a Western diet | Mice on HFD | American Ginseng | NR | Decreased tumour volume and number | Decreased IL-1α, IL-1β, IL6 and TNF-α |
| Clinical probiotics studies: | ||||||
| Gao et al. 2017 [74] | CRCs patients underwent radical surgery and scheduled for further surgery | RCT Placebo (n = 11) Probiotic (n = 11) Controls (n = 11) | Bifidobacterium longum, L. acidophilus and Enterococcus faecalis | Increased Enterococcus. Decreased Peptostreptococcus and Fusobacterium | NR | NR |
| Zhang et al. 2012 [105] | CRCs patients scheduled for surgery | RCT Placebo (n = 30) Preoperative probiotic (n = 30) | Bifidobacterium longum, L. acidophilus and Enterococcus faecalis | Increased preoperative ratio of Bifidobacterium/E. coli | Decreased incidence of post-surgery infections | Increased IgG in serum. Decreased endotoxins, IL-6, and CRP |
| Aisu et al. 2015 [107] | CRCs patients scheduled for surgery | Clinical study Control (n = 81) Probiotic (n = 75) | Enterococcus faecalis (T110), Clostridium butyricum (TO-A) and Bacillus mesentericus | NR | NR | Decreased incisional infections and reduced post-surgical acute inflammation |
| Liu et al. 2011 [106] | CRCs patients scheduled for surgery | Randomized double-blind clinical study. Placebo (n = 50) Probiotic (n = 50) | L. plantarum, L. acidophilus and Bifidobacterium longum | Increased Bifidobacterium and Lactobacilli. Decreased Enterobacteriaceae, Pseudomonas and Candida | NR | Increased claudin-1, and occludin |
| Gianotti et al. 2010 [125] | CRCs patients scheduled for surgery | Randomized double-blind clinical study. Placebo (n = 10), Probiotic at a dose of 107 (n = 11) Probiotic at a dose of 109 (n = 11) | Bifidobacterium longum (BB536) and L. johnsonii (La-1) | La-1 increased La-1 in mucosa and feces. Decreased Enterobacteriaceae | NR | Increased lymphocytes CD3+, CD4+, and CD8+ |
| Bajramagic et al. 2019 [42] | Stage III CRCs | RCT of 78 patients with CRCs who undergo for surgery | L. acidophilus, L. casei, L. plantarum, L. rhamnosus B. lactis, B. bifidum, B. breve and Streptococcus thermophilus | NR | NR | Decreased number of complications associated with CRCs surgeries |
| Clinical prebiotics studies: | ||||||
| Zhu et al. 2021 [118] | CRCs patients | CRCs versus healthy patients undergoing colonoscopies | Evodiamine | Increased E. faecalis and E. coli. Deceased Campylobacter, Bifidobacterium and L. in CRCs | Decreased proliferation, p-STAT3 after incubation with evodiamine in cancer cells | Increased pSTAT3 in cancerous versus peritumour biopsies |
| So et al. 2021 [108] | Patients at high risk of CRCs | Randomized, double-blind, controlled trial (placebo group 19 and treatment group 20) | Rice bran | Increased Firmicutes and Lactobacillus | NR | NR |
| Xie et al. 2019 [109] | CRCs patients requiring radical surgery | Randomized double-blind clinical study for CRCs (control 70 and prebiotics 70) | Fructo-oligosaccharides, xylo-oligosaccharides, and polydextrose | Increased Bifidobacterium and Enterococcus, and decreased Bacteroides before surgery. Increased Escherichia-Shigella, and decreased Enterococcus after surgery | NR | Increased IgG, IgM before surgery. Increased IgG, T lymphocytes CD3+, CD8+ and B lymphocytes CD19+ after surgery |
| Clinical symbiotics studies: | ||||||
| Polakowski et al. 2019 [110] | CRCs stages I-III scheduled for surgery | Double-blind, randomized, placebo-controlled clinical study (placebo 36 and treatment 37) | Maltodextrin as placebo and treatment with Simbioflora® (fructo-oligosaccharide, plus L. acidophilus, L. rhamnosus, L. casei and B. lactis) | NR | 3 deaths in the control group and no death in the treatment group | Decreased plasma IL-6, post- surgery infections, antibiotic use and hospital stay |
| Hibberd et al. 2017 [84] | Stage I–III CRCs undergoing surgery | Clinical trial (21 healthy controls, 15 CRCs 8 non-intervention 7 probiotic) | B. lactis, L. acidophilus and inulin | Increased Firmicutes, Clostridiales spp. and Faecalibacterium | NR | NR |
| Clinical pre-, pro- and symbiotic studies: | ||||||
| Worthley et al. 2009 [126] | CRCs prevention in healthy subjects | Randomized double-blind clinical study with crossover treatments | Prebiotic (resistant starch) probiotic (Bifidobacterium lactis) or combination of both (symbiotic) | Symbiotic increased Lachnospiraceae spp. | NR | No changes in fecal ammonium, SCFAs, cytokines and/or epithelial changes |
| Clinical food studies: | ||||||
| Brown et al. 2017 [127] | CRCs patients undergoing surgery | Randomized clinical study (controls 10 and normal controls 9) | Rice bran | NR | NR | Increased carbohydrate, lipid, amino acid, and vitamin metabolism |
| Nunez-Sanchez et al. 2017 [111] | CRCs patients scheduled for surgery | Randomized clinical study (controls 10 and patients 35) | Pomegranate extract with high ellagitannin content | NR | NR | Decreased CD44+ in adjacent both malignant and healthy tissue |
| Nunez-Sanchez et al. 2015 [112] | CRCs patients scheduled for surgery | Randomized double-blind clinical study (controls pomegranate extract #1 and pomegranate extract #2) | Pomegranate extract with high ellagitannin content | NR | NR | Modulation of miRNAs |
[i] A. muciniphila – Akkermansia muciniphila, AOM – azoxymethane, APC – adenomatous polyposis coli, B. – Bifidobacterium, CEA – carcinoembryonic antigen, CD44+ – multifunctional cell surface molecule involved in cell proliferation, COX-2 – cyclooxygenase-2, CRCs – colo-rectal cancers, CRP – C-reactive protein, CYP – Cyp-Express-1A1 or 1B1-Cytochrome P450, DHM – dihydromyricetin, DSS – dextran sodium sulfate, E. – Enterococcus, EPA – eicosapentaenoic acid, FOLFOX – 5-fluorouracil, leucovorin, and Lactobacillus casei oxaliplatin, GPR43 – G-protein-coupled receptor-43, HFD – high fat diet, Ig – immunoglobulin, IL-2 – interluekin-2, Ki-67-positive cells – is often correlated with the clinical course of cancer, L. – Lactobacillus, LFD – low fat diet, MD – Mediterranean diet, miRNAs – micro-RNAs that act as epigenetic regulators, LMW – low molecular weight, NR – not reported, PD-1 – programmed cell death protein-1, pSTAT3 – over expression of STAT3protein and is associated with poor prognosis, PUFA – polyunsaturated fatty acids, RCT – randomized controlled trial, SCFAs – short chain fatty acids, SLC5A8 – carrier family 5 member 8, TLR4 – Toll-like receptor 4, TNF – tumour necrosis factor.
The quality of the reviewed studies was assessed using the CONSORT and STROBE checklists. The CONSORT checklist is a 25-item checklist focusing on the article/study design, analysis, and interpretation. The STROBE checklist is a 22-item checklist evaluating different sections/parts of the observational studies.
Discussion
The microbiota works as a natural defence, and it modulates the host’s immune response against tumour development and progression [8]. Although the value of nutrition is frequently underestimated, the available data showed that nutrition is crucial in shaping the gut microbiota. Therefore, this systematic review aimed to detect the effect of microbiota modulation using probiotics, prebiotics, symbiotics, and natural changes on CRCs.
Lactobacillus combined with Bifidobacterium is one of the most studied probiotic combinations in CRCs.
Although Yuan et al. [66] found that the combination of Bifidobacterium and Lactobacillus cannot improve the response to 5-FU treatment in CRCs, Lactobacillus acidophilus combined with Bifidobacterium longum and Enterococcus faecalis (Bifico cocktail) was approved in China in preclinical studies for reduction of colon tumour growth [67, 68].
Moreover, the combination of 3 strains of Lactobacillus with one strain of Bifidobacterium decreased the incidence of CRCs in a preclinical study [69].
The use VSL#3 (Lactobacillus, Bifidobacterium, and Streptococcus) with a Western diet and metformin decreased the development of CRCs in a murine model [70] compared to VSL#3 alone [71].
Chang et al. [72] reported improved microbiota and inflammatory parameters when Lactobacillus casei was used in combination with FOLFOX (5-FU, leucovorin, and oxaliplatin) in CRCs associated with colitis in an animal model, Chang et al. [72] found that Lactobacillus casei inhibits healthy intestinal epithelial death while FOLFOX increases malignant cell apoptosis.
Lactobacillus casei BL23 strain were protective against CRCs in a mouse model, it downregulates inflammatory cytokines (interleukin-22), and it has an immunomodulatory and anti-proliferative effect [73].
Gao et al. [74] found the Lactobacillus reuteri (i.e. a histamine-producing probiotic) reduced the cancer-associated inflammatory cytokines and the number and size of CRCs in an animal model.
The therapeutic effect of interleukin-2 (IL-2) as an immunotherapy was significantly augmented by the oral use of Akkermansia muciniphila (A. muciniphila) in CRC-bearing mice [75].
Shi et al. [75] found that tumour volume decreased and survival rate increased in mice with CRCs after the use of IL-2 and A. muciniphila. Therefore, the SCFAs producing probiotics and A. muciniphila could have a useful therapeutic effect for patients with CRCs.
Pectin is SCFAs producing prebiotic [76], reduces the ammonia [77], and improves glucose metabolism [78]. Pectin is a metabolite derived from plant wall metabolite, which serves as an energy source for SCFA-producing bacteria after fermentation in the colon [79]. Moreover, pectin is protective against colorectal, prostate, and breast cancers [80]. Pectin increases the SCFA-producing bacteria (i.e. acetate) and increases the expression of interferon-γ-producing TCD8+ [81].
Galacto-oligosaccharides that are originated from lactulose reduced the CRC development and modulated the microbiota in preclinical studies [82]. Fructo-oligosaccharides (inulin) reduced the volume of implanted colonic tumours in an animal model [83]. They inhibit the tumour development through butyrate production and microbiota modulation [84].
The normal colonocytes use butyrate as the main energy source [85, 86]. Additionally, butyrate inhibits histone deacetylase and increases malignant colonocytes apoptosis [34].
Wu et al. [87] found that polymethoxyflavones were effectively prevent carcinogen-induced CRCs in an animal model through modulation of gut microbiota and increased butyrate-producing bacteria.
Hu et al. [88] found that resistant starch was able to protect against colitis-associated CRCs in rats by increasing SCFA-producing bacteria. Zhang et al. [89] found that Canmei herbal formula (containing Mume sieb, Marci Hieronymi, and more than 41 active ingredients – mainly DL-arginine, L-carnitine, and L-tyrosine) reduced the colitis associated with CRCs in an animal model through modulation of the colon microbiota.
Symbiotics can increase the efficacy of CRC treatments. They decrease the invasiveness of CRCs and increase the SCFA-producing microbiota [90].
Zheng et al. [91] found that symbiotics containing dextran and Clostridium butyricum (C. butyricum) regulate the gut microbiota and increase the SCFA-producing microbiota. Further studies regarding the ideal symbiotic and its proper therapeutic dose for CRCs are warranted.
Prebiotics can be extracted from mushrooms, green tea [92], and fruits [93]. Probiotics can be extracted from fruit [94], dairy products (i.e., yogurt, milk, and cheese) [95], and dairy products fermentation [96]. A high-soluble-fibre diet can inhibit intestinal mucosal erosions, decrease its degradation, and enhance the intestinal mucosal barrier [3]. Anthocyanins (i.e. blackberries) decrease local colonic inflammation, improve the gut microbiota, and reduces the incidence of CRCs [93, 97].
Marine extract containing certain shellfish may have anticarcinogenic effects [98, 99]. Terasaki et al. [100] found that fucoxanthin (algae-derived product) present in Undaria pinnatifida decreased the number of CRC-adenocarcinomas in an animal model. Nutmeg can decrease the development of adenomatous polyposis coli-induced CRCs (API-induced CRCs) [101].
The Mediterranean diet prevents certain types of cancer [102] and reduces the incidence of CRCs by 11% [103]. The Mediterranean diet combined with a high-fat diet improves the gut microbiota [104].
Bajramagic et al. [42], in a randomized controlled trial (RCT) including 78 patients, studied the effect of probiotics in reducing the post-operative side effects after surgeries for stage III adenomatous CRCs. They found that the probiotics mixture containing Lactobacillus, Bifidobacterium, and Streptococcus strains decreased the common post-operative side effects after CRC surgery, especially post-operative paralytic ileus.
Zhang et al. [105] found that a probiotic mixture containing Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis in CRCs decreases the Fusobacterium (Gram-negative, anaerobic bacteria) associated with CRCs and decreases post-operative infection after CRC surgeries.
The probiotic containing L. plantarum, L. acidophilus, and Bifidobacterium longum reduces post-operative diarrhoea, fever, and infection [106].
The Lactobacillus johnsonii (La1) reduces the pathogenic microorganisms and colonic inflammation after CRC surgeries [5].
The pre-operative use of probiotic mixture containing Enterococcus, Clostridium, and Bacillus significantly reduced post-operative incisional infections after CRC surgeries [107].
The intake of phytochemicals increases the Firmicutes and Lactobacillus levels and decreases the risk of CRCs [108].
Xie et al. [109] in a RCT found that pre-operative use of non-digestible oligosaccharides in patients who underwent radical surgeries for CRCs improved their immune response. Xie et al. [109] explained the improved immune response by the positive effect of non-digestible oligosaccharides on the gut microbiota.
The symbiotic (simbioflora®) containing oligosaccharides, Lactobacillus, and Bifidobacterium lactis strains significantly reduced post-operative infection after CRC surgeries [110].
The Bifidobacterium, Lactobacillus, and inulin strains improved SCFA-producing microbiota in patients who underwent stage I–III CRC surgeries [84].
Nuñez-Sánchez et al. [111, 112] found that intake of pomegranate extract changed the expression of certain genes [111] and microRNAs [112] in CRCs, highlighting the role of diet modification in changing the gene expression in CRCs.
This systematic review found the gut microbiota metabolites, SCFAs (i.e. acetate and butyrate) produced by the lactic acid-producing, and Bifidobacteria have a protective effect against CRCs. SCFAs reduce the inflammatory cytokines, inhibit colonocytes proliferation, and promote malignant cell apoptosis. Butyrate maintains mucus membrane barrier integrity, regulates mucosal cell junction occlusion, and reduces intestinal mucosal inflammation. The beneficial effect of pro-, pre-, and/or symbiotics on CRCs should be confirmed in future studies.
Conclusions
Prebiotics (i.e. inulin and resistant starch, SCFA producers) and consumption of unprocessed plant products are useful for developing and maintaining healthy gut microbiota. The pro-, pre-, and/or symbiotics may be useful when carefully selected for CRC patients, to restore the beneficial gut microbiota and support the treatment efficacy. The beneficial effect of pro-, pre-, and/or symbiotics on CRCs should be confirmed in future studies.