Purpose
Carcinoma cervix is a common cancer in developing countries. In India, carcinoma of uterine cervix is the second most common cancer among females, and the third most common cancer overall [1]. Concurrent chemo-radiotherapy (CCRT) with weekly cisplatin, followed by brachytherapy is the current standard of care for locally advanced cervical carcinoma (LACC) [2-6]. Radical radiotherapy comprises of external beam radiotherapy (EBRT) and brachytherapy (BT).
Traditionally, whole pelvic radiation therapy has been delivered with four-field box technique or three-dimensional conformal radiotherapy (3D-CRT), but these techniques are associated with significant rates of gastrointestinal (GI), genitourinary (GU), and hematologic toxicities [7]. In patients treated with CCRT, acute grade > 2 GI and GU toxicities range from 20-30%, and grade > 2 hematological toxicities occur in the range of 30-40% [8]. Intensity-modulated radiation therapy (IMRT) leads to a significant reduction in the rates of grade > 2 acute GI and GU toxicities as well as late GI and GU toxicities, as compared with conventional techniques, without affecting clinical outcomes [9-15]. Organ motion and tumor regression during radiotherapy pose significant problems for IMRT planning, potentially leading to target miss with smaller applied planning target volume margins. Larger margins lower the degree of organ sparing possible with IMRT. Hence, incorporation of image guidance with IMRT may be needed to tackle the inter- and intra-fraction movements. Image guidance using daily kilovoltage cone-beam computed tomography (kV-CBCT) with IMRT (IG-IMRT) is an effective approach in optimizing the therapeutic ratio in LACC [16].
Brachytherapy either in the form of intracavitary radiation therapy (ICRT) or interstitial brachytherapy (ISBT), is an integral part of radical radiotherapy for carcinoma cervix. Although conventional X-ray-based planning may yield descent clinical outcome, toxicities (in particular, late) may decrease quality of life of patients. With the emergence of Groupe Européen de Curiethérapie and European Society for Radiotherapy & Oncology (GEC-ESTRO) recommendations, volume-based image-guided brachytherapy has become feasible [17, 18]. Many studies support the use of image-guided brachytherapy (IGBT) to improve therapeutic ratio in cervical cancer [19-22]. Magnetic resonance imaging (MRI)-based IGBT has shown promising benefits for cervical cancer patients, according to EMBRACE-I and retro-EMBRACE studies [21, 22]. EMBRACE-I has reported an impressive 5-year local control and survival rate of 92% and 74%, respectively, with fewer severe morbidity rates using MRI-IGBT [21].
Magnetic resonance imaging is superior to CT for target volume delineation during brachytherapy [23]. However, it may not be possible to perform MRI in every BT session in resource-constrained settings, such as in India. Therefore, first fraction under MRI guidance and subsequent fractions with CT (hybrid imaging technique of IGBT) can be a reasonable alternative approach [24]. With the development of radiotherapy techniques, such as IG-IMRT and IGBT, combinations of these techniques of irradiation need to be studied, as there is a paucity of evidence evaluating this treatment concept. Therefore, we aimed to assess the acute toxicity, loco-regional control, and late toxicities in LACC patients treated with IG-IMRT and IGBT.
Material and methods
This was a prospective, single-arm interventional study conducted in a tertiary care center in India from January 2020 to May 2022 (CTRI/2020/08/027434). This study protocol was approved by the institutional ethical committee, with approval No.: IEC No 51/19.
Biopsy-proven locally advanced cervical carcinoma stage IIA2-IIIC1 (FIGO 2018) patients, aged between 18 and 65 years, with a Karnofsky performance status (KPS) ≥ 70, hemoglobin level ≥ 10 g/dl, total leukocyte count ≥ 4,000/mm3, platelet count ≥ 100,000/mm3, creatinine clearance level ≥ 60 ml/min, and normal liver function tests were eligible for the current study. Patients with non-squamous histology, para-aortic lymph nodes, distant metastases, or synchronous/metachronous malignancy were excluded. Written informed consent was obtained from all patients. Pre-treatment assessment included detailed history, gynecological examination, complete blood counts, liver and kidney function tests, MRI of the abdomen and pelvis, and chest X-ray. 18FDG PET-CT (18F-fluorodeoxyglucose-positron emission tomography), cystoscopy, and proctosigmoidoscopy were performed whenever clinically indicated.
Whole pelvic IG-IMRT
All patients received EBRT of 45 Gy in 25 fractions over five weeks (IIIC1 patients received 55 Gy in 25 fractions as simultaneously integrated nodal boost) using IG-IMRT. Contrast-enhanced CT simulation was performed in all cases. Bladder filling protocol (after voiding, patients were asked to drink 500 ml of plain water 30 minutes before treatment and to hold the urine) was followed at the time of simulation and subsequently before each fraction of EBRT. Radio-opaque vaginal marker was placed at the most distal portion of the cervical growth. Small radio-opaque marker was also placed at level of the perineum. After intravenous contrast, 3 mm CT images were scanned from the upper border of the T10 vertebral body to the mid-thigh in a supine position with a knee rest. CT images were transferred to treatment planning system (TPS, Monaco version 5.11.02) using DICOM 3.0 protocol. Clinical target volume (CTV) comprised of primary tumor and regional lymphatics. Primary CTV incorporated the entire uterus, cervix, parametrium, and vagina up to 2 cm below the vaginal extent of the disease. Nodal CTV included the common iliac, external and internal iliac, obturator, and pre-sacral nodal regions. All lymph nodal regions were contoured according to Taylor et al. guidelines [25]. Planning target volume (PTV) was defined as the primary CTV with 1 cm isotropic margin, and nodal CTV with a 0.7 cm isotropic margin. Bowel bag, rectum, bladder, and femoral heads were contoured as organs at risk (OARs). The bowel bag was contoured as the abdominal cavity from the recto-sigmoid junction up to 3 cm above the superior most section of the PTV contour.
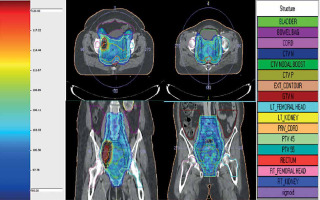
IMRT planning constraints used for target coverage were as follows: 95% prescription isodose surface to encompass ≥ 95% of PTV and maximum dose of ≤ 107% (contained within PTV). Dose constraints for normal tissues were as follows: (1) Bowel bag: volume receiving 40 Gy (V40Gy) < 100 cc and 30 Gy (V30Gy) < 350 cc. In cases of nodal boost, V40Gy < 250 cc and V30Gy < 500 cc; (2) Rectum: V40Gy < 85% and V30Gy < 95%, Dmax < 105% (in case of IIIC1 patients); (3) Bladder: V40Gy < 75%, and V30Gy < 85%, Dmax < 105% (in case of IIIC1 patients); (4) Femoral heads: maximum dose (Dmax) ≤ 50 Gy; (5) Sigmoid: maximum dose ≤ 48 Gy. Plans were evaluated using dose-volume histograms (DVH), planar isodose display, and 3D isodose display (Figure 1). Conformity index (TV ref [total volume covered by the referenced isodose]/TV [volume of total PTV]), and homogeneity index (D2% [dose received by 2% volume of PTV]/D98% [dose received by 98% volume of PTV]) were also evaluated. Treatment was delivered using Elekta Infinity linear accelerator, with MLC leaf width of 1 cm at the isocenter by volumetric modulated arc therapy (VMAT). Treatment verification was performed daily with kV-CBCT before treatment in all patients, and on-line corrections were made prior to treatment delivery. Concurrent chemotherapy was given with weekly intravenous cisplatin of 40 mg/m2. Dose was modified according to weekly assessment of creatinine clearance level.
Image-guided brachytherapy
Brachytherapy was started within a week of completion of IG-IMRT. Four fractions, once or twice weekly (to optimize overall treatment time within 8 weeks), with dose of 7 Gy at each session using high-dose rate (HDR) intracavitary brachytherapy were delivered to all patients. First fraction was applied under MRI guidance, and subsequent three fractions were performed under CT guidance. All patients had the bowel and part preparation one day prior to implant procedure.
IGBT application procedure
Complete gynecological examination was done before each session of IGBT. All patients underwent first fraction of IGBT under spinal anesthesia. In subsequent fractions, if the implant procedure was not deemed to require spinal anesthesia, the procedure was performed under short general anesthesia or conscious sedation. Patients were catheterized, and 7 cc of normal saline was filled in the bulb of Foley’s catheter. Additionally, for CT-planned fractions, a normal saline solution (18 cc) plus 2 cc of contrast media was instilled into the bladder before imaging, and 20 cc of normal saline was instilled into the bladder before delivery of brachytherapy treatment (after emptying bladder with asepto syringe before instillation at each instance). Cervical os was dilated using serial Hegar’s dilators till size 10. We used the least possible sized dilators to avoid injury and uterine perforation along with real-time trans-rectal ultrasonography during the procedure of applicator placement. Uterine canal was sounded, and uterine canal length was gauged. MR-compatible modified Fletcher-Suit tandem (Elekta) of suitable length, and two appropriate size ovoids were placed in all fractions of IGBT. Following this, adequate packing was done.
Imaging and planning of IGBT
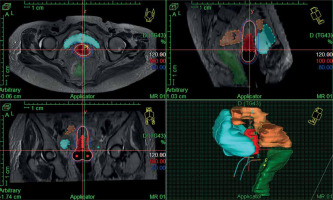
In the first fraction of IGBT, T2-weighted sequences in para-axial, para-sagittal, and para-coronal planes were obtained on 3T MRI (GE Healthcare) with a pelvic coil, which were used for IGBT planning during the first session. Subsequent three BT sessions were done under CT guidance using CT scanner (Siemens Somatom Sensation Open). MRI/CT images were acquired without intravenous contrast at 3 mm slice thickness covering an anatomical region extending from the L4-L5 vertebrae to the ischial tuberosity. Then, the MR/CT images were imported into Oncentra TPS version 4.5.3 (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden). Delineation of HR-CTV during first fraction was performed according to GEC-ESTRO recommendations [17, 18]. Delineation of HR-CTV was based on MRI information at the time of first fraction supported by clinical examination findings. HR-CTV volume included the entire cervix, gross disease at the time of IGBT, and grey zones on MRI. HR-CTV was delineated on CT images for subsequent fractions (CT1, CT2, CT3) according to Viswanathan et al. guidelines [26]. HR-CTV delineation on CT was based on clinical examination at the time of brachytherapy as well as information from MRI done at the first fraction of IGBT. Volumes and dimensions of HR-CTV were noted at each fraction of IGBT. Prescribed dose was 7 Gy × 4 fractions to HR-CTV (volume-based plan) for all patients (Figure 2), and DVHs for HR-CTV and OARs were evaluated. D90 (minimum dose covering 90% of target volume) of HR-CTV was recorded for all patients. D2cc (minimum doses calculated at the most irradiated 2 cc volumes) of the bladder, rectum, and sigmoid colon were assessed. The total reference air-kerma (TRAK) values and point-A doses for each fraction were also documented. Cumulative doses to HR-CTV and OARs (WP-IMRT and IGBT) were converted to equivalent doses in 2 Gy per fraction (EQD2) using linear-quadratic model (with α/β value of 10 for tumor, and α/β value of 3 for OARs) [27].
EQD2 of WP-IMRT and IGBT were summed up to evaluate the volume-based plan with regards to DVH constraints corresponding to a prescribed EQD2 dose of at least 80 Gy α/β 10 (D98% of WP-IMRT plus D90% of IGBT). For OARs, cumulative total EQD2 dose (D2% of WP-IMRT plus D2cc of IGBT) were aimed to be ≤ 65 Gy α/β 3 for the rectum, ≤ 70 Gy α/β 3 for the sigmoid, and ≤ 80 Gy α/β 3 for the bladder, respectively. IGBT treatment was delivered with iridium-192 source (MicroSelectron HDR, Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden). After completion of the treatment, applicators were removed, and routine antibiotics and symptomatic medications were advised to avoid infections and patients’ discomfort.
Follow-up and evaluation of toxicity
Patients were assessed weekly throughout the treatment and until 90 days after beginning of the treatment for acute GI, GU, vaginal and hematologic toxicities according to common terminology criteria for adverse events version 5.0 [28]. Late toxicities were evaluated during follow-up according to the Radiation Therapy Oncology Group criteria. After treatment completion, patients were followed up every month for 6 months, and then once every 3 months to date. At each follow-up visit, complete history was obtained and per vaginal examination was performed. MRI scan of the abdomen and pelvis was obtained at 3 months post-treatment in all patients, and subsequent imaging was done only in case of clinical suspicion.
Statistical analysis
Sample size calculation for the present study was not done, and the sample size was limited to 25 patients based on the available resources. The primary endpoint was acute toxicity and secondary endpoints were 2-year local, loco-regional, distant control, and late toxicities. Quantitative variables were described in median with range. Local control (LC) and loco-regional control (LRC) were defined as the absence of any disease clinically or on imaging at primary site and primary/nodal sites, respectively, and was calculated from the date of start of treatment. Loco-regional recurrence-free survival (LRRFS) was estimated using Kaplan-Meier method. All data were analyzed using SPSS statistical software package for Mac (version 23.0; IBM, Armonk, NY, USA).
Results
All patients completed the treatment, and none defaulted during the treatment; therefore, complete analysis was performed on 25 patients. Patients’ characteristics are summarized in Table 1. The median EBRT dose was 45 Gy (range, 45-55 Gy). All patients received concurrent chemotherapy with a median number of 5 cycles (range, 4-5 cycles). The median gap between IG-IMRT completion and first fraction of IGBT was 5 days (range, 4-7 days). The median overall treatment time was 8 weeks (range, 7-10.5 weeks). One patient became infected with coronavirus disease (COVID-19), and completed treatment in 10.5 weeks. The median gap between four fractions of IGBT was 4 days (range, 3-6 days). Sixteen patients (64%) showed a complete response, and nine patients (36%) had residual disease at first fraction of IGBT. All the patients received intracavitary brachytherapy, and none of them required a combined intracavitary and interstitial brachytherapy.
Table 1
Patient characteristics of entire cohort
Dosimetric outcome
The median coverage of 95% of PTV by 95% of prescribed dose was 99.18% (range, 96.85-99.80%). The median conformity index and homogeneity index were 0.98 (range, 0.96-0.99) and 0.95 (range, 0.93-0.96), respectively. DVH parameters for OARs are presented in Table 2. The median dose to 98% of PTV, 2% of the bladder, 2% of the rectum, and 2% of the sigmoid colon were 42.8 Gy (range, 42.3-43.4 Gy), 45.6 Gy (range, 45.3-47.8 Gy), 45.2 Gy (range, 45.1-46.2 Gy), and 47.9 Gy (range, 46.3-48.8 Gy), respectively.
Table 2
Dose volume parameters for organs at risk during image-guided intensity-modulated radiation therapy (IG-IMRT)
Table 3 shows the comparison of HR-CTV doses, dimension, volumes, point-A doses, and TRAK for different fractions of IGBT. The median per fraction doses of IGBT for D2cc bladder, D2cc rectum, and D2cc sigmoid colon were 5.01 Gy (range, 3.5-5.8 Gy), 3.4 Gy (range, 2.2-5.1 Gy), and 3.7 Gy (2.2-5.2 Gy), respectively. The median cumulative doses in terms of EQD2 of IGBT plus WP-IMRT for D90 HR-CTV, D2cc bladder, D2cc rectum, and D2cc sigmoid colon were 87 Gy (range, 81.8-95.8 Gy), 72.95 Gy (range, 65.3-83.2 Gy), 63.1 Gy (range, 57.8-68.8 Gy), and 65.6 Gy (range, 61.2-75.2 Gy), respectively. Cumulative EQD2 of < 85 Gy for D90 HR-CTV, > 80 Gy for the bladder, > 65 Gy for the rectum, and > 70 Gy for the sigmoid were recorded in 3 (12%), 2 (8%), 1 (4%), and 2 (8%) patients, respectively. Cumulative EQD2 to HR-CTV D90 doses in patients with partial responses (n = 7) were > 85 Gy.
Table 3
Comparison of high-risk clinical target volume (HR-CTV) doses, dimension, volumes, point-A doses, and TRAK for different fractions of brachytherapy
Toxicity analysis
None of the patients reported grade 3 or 4 acute toxicity. Grade 1 and 2 diarrhea, and grade 1 cystitis were reported in 4 (16%), 3 (12%), and 2 (8%) patients, respectively. Grade 1 and 2 anemia, and grade 1 and 2 dermatitis were observed in 3 (12%) and 2 (8%), and 3 (12%) and 3 (12%) patients, respectively. Acute grade 1 and 2 vaginal inflammation was observed in 2 (8%) and 1 (4%) patients, respectively. Acute toxicities in patients treated with SIB boost (IIIC1) vs. non-SIB boost were grade 2 radiation dermatitis 28.5% vs. 5.5%, grade 2 diarrhea 28.5% vs. 5.5%, and grade 1 anemia 42.8% vs. 0%, respectively. Late grade 1 bladder toxicity was observed in 1 (4%) patient. Grade 1 vaginal stricture and grade 1 vaginal dryness were seen in 2 (8%) and 1 (4%) patients, respectively. No late lower GI or skin toxicity were reported. None of the patients reported grade 2 or higher late toxicities.
Clinical outcome
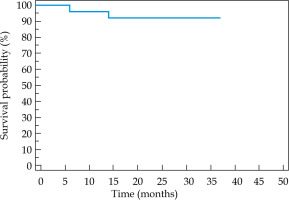
At a median follow-up of 29.5 months (range, 25-37 months), two patients developed local recurrence confirmed by a biopsy: one after 6 months and another after 14 months of the treatment completion. Both patients were given palliative chemotherapy, and both had stable disease at the time of last follow up. One patient died from COVID-19 pneumonitis at 25 months after the treatment. 2-year local control, loco-regional control, metastasis-free survival, and overall survival rates were 92%, 92%, 100%, and 96%, respectively. 2-year LRRFS was 92% (Figure 3).
Discussion
The use of WP-IMRT for intact cervical carcinoma has increased since the last decade, resulting in adequate acute toxicity profile and clinical outcome [9-15]. Dosimetrically, IMRT has been found to be superior to conventional techniques due to sparing of OARs and more conformal dose to primary target [14]. In our study, we adopted CTV and OARs dose constraint of the EMBRACE-II protocol and in majority of patients (92%), dose constraints were achieved and resulted in excellent toxicity profiles, supporting the validation of dose constraints set by the EMBRACE-II study [29]. IMRT technique in LACC is associated with lower incidence of acute grade 3-4 GI (range, 4-6%) and GU toxicity (range, 2-7%) [9, 13-14]. In our study, none of the patients reported grade 3-4 acute GI and GU toxicities, which can also be attributed to smaller sample size or to the use of daily kV-CBCT. With the utilization of IG-IMRT, we observed a reduction in acute and late GI toxicities in comparison with other LACC patients treated with 3D-CRT at our institute (unpublished data). The incidence of late GI toxicity is significantly lower with IMRT as compared with conventional radiotherapy [9, 11, 15]. In the present study, none of the patients have reported grade > 1 late GI and GU toxicities. Late toxicities are important parameters for determining the quality of life of patients, but are generally under-reported. Also, late toxicities usually begin after 6 months of treatment completion and continue to increase with further follow-up. Longer follow-up of our study cohort would more accurately determine late toxicities.
IGBT in LACC has shown improvement in dose volume parameters and clinical outcome owing to enhanced HR-CTV and reduced OARs doses [19-23]. In our study, we found similar results as in EMBRACE studies [21], with median EQD2 of D90 HR-CTV, D2cc bladder, D2cc rectum, and D2cc sigmoid colon as 87 Gy (range, 81.8-95.8 Gy), 72.95 Gy (range, 65.3-83.2 Gy), 63.1 Gy (range, 57.8-68.8 Gy), and 65.6 Gy (range, 61.2-75.2 Gy), respectively, which resulted in decent LRC and reduced treatment-related morbidity. The median HR-CTV volumes for the first fraction (MRI), second fraction (CT1), third fraction (CT2), and fourth fraction (CT3) were 21.06 cc (range, 17.2-30.0 cc), 28.4 cc (range, 21.74-34.4 cc), 27.05 cc (range, 22.7-32.5 cc), and 25.6 cc (range, 20.05-31.03 cc), respectively. The HR-CTV volumes in CT-planned fractions were larger as compared with the MRI-planned fractions, and this slight overestimation have been also reported by previous studies [24].
The combination of advanced techniques of IMRT and IGBT in patients of LACC is a new approach, which may improve therapeutic ratio by decreasing treatment-related morbidity and increasing loco-regional control. However, few studies have reported outcomes of patients with this approach [30-32]. Although, retrospective studies have reported the clinical outcome of WP-IMRT with IGBT [30, 31], they are limited by a prospective robust toxicity assessment and quality control. Retrospective studies [30, 31] have reported a higher > grade 2 late GI/GU toxicities ranging from 8.5% to 11%. A prospective study by Tharavichitkul et al. [32] reported a higher acute grade 1-2 GI (60% vs. 28%) and GU (53% vs. 28%) toxicities as compared with the present study. This may be due to the use of larger PTV margins (range, 0.7-1.5 cm) in their research as compared with the current study (range, 0.7-1.0 cm). Moreover, the authors did not report dosimetric parameters of the small bowel/bladder during EBRT, and we do not know if these higher toxicities may have been due to higher doses to OARs in their study. Table 4 summarizes the clinical outcomes of reported studies using WP-IMRT and IGBT for LACC.
Table 4
Results of published studies on IMRT and IGBT in carcinoma cervix, and comparison with the current study
| Study [Ref.] | Study design | No. of patients treated with IG-IMRT + IGBT | Median follow-up duration | Clinical outcome of (WP-IMRT → IGBT) | Toxicity profiles of (WP-IMRT → IGBT) |
|---|---|---|---|---|---|
| Lin et al. [30] | Retrospective 2 arms (WP-IMRT → IGBT -CT/MRI-based) vs. (WP-CRT → 2D-BT) | 300 (total n = 600) | 7.2 years | 5-year FFR: 65% 5-year CSS: 69% 5-year OS: 61% | > grade 2 late GI/GU toxicities: 11% |
| Chen et al. [31] | Retrospective (WP-IMRT → IGBT MRI-based) vs. (WP-IMRT → 2D-BT) | 117 (total n = 253) | 47 months | 4-year LRFS: 87% 4-year OS: 76% | > grade 2 late GI: 8.5% > grade 2 late GU: 1.7% |
| Tharavichitkul et al. [32] | Prospective single-arm WP-IMRT → IGBT (CT-based) | 15 | 14 months | LRC: 93% MFS: 100% OS: 93% | Skin ≤ grade 2: 6% GI ≤ grade 2: 60% GU ≤ grade 2: 53% No grade > 2 toxicity seen Late vaginal stenosis grade 1: 6% |
| Present study | Prospective single-arm WP-IMRT → IGBT (MRI/CT-based) | 25 | 29.5 months | LRC: 92% MFS: 100% OS: 96% | Skin ≤ grade 2: 20%, GI ≤ grade 2: 28% GU ≤ grade 2: 8% Late: GU, grade 1: 4%, vaginal stricture grade 1: 8% |
[i] WP-IMRT – whole pelvic intensity-modulated radiotherapy, IGBT – image-guided brachytherapy, WP-CRT – whole pelvic conventional radiotherapy, 2D-BT – 2 dimensional brachytherapy, FFR – freedom from relapse, CSS – cancer-specific survival, OS – overall survival, GI – gastrointestinal, GU – genitourinary, LRC – loco-regional control, MFS – metastasis-free survival
Not all LACC patients would respond to the current protocol of IG-IMRT and IGBT, and this may be due to other molecular and biological factors. Ongoing research may help to better stratify these patients for treatment escalation or de-escalation. Chopra et al. reported the international collaborative translational study of the risk stratification of LACC patients treated with CCRT [33]. The authors reported that although p16+ve cervical cancer patients presented with higher nodal positivity rate and more necrosis on MRI, these patients were also more likely to present with lower HR-CTV volume at the time of brachytherapy, showing their exquisite radiosensitivity. P16-ve cervical cancer patients in this study had higher chances of HR-CTV volume at brachytherapy of > 40 cc and poorer pelvic control rates. L1CAM expression of > 50% was also reported to be associated with a poorer pelvic control rates [33]. More biomarker and translational studies in future may help in better risk stratification and further personalization of management of LACC patients.
There is an excess of data on the role of IMRT and IGBT in LACC patients [9-15, 18-24], but the current investigation is one of the few studies that has evaluated the combined effect of IG-IMRT with daily kV-CBCT and IGBT using hybrid imaging technique in LACC. Results of the EMBRACE-II study [29] are eagerly awaited and till then, the results of our study may be reassuring in favor of combination of IG-IMRT and IGBT. We were able to demonstrate that the dosimetric sparing of OARs during EBRT and IGBT yields excellent acute toxicity and clinical outcome in LACC patients. Additionally, the use of MR-IGBT at first fraction and CT-based IGBT in subsequent fractions may be a resource-sparing approach in low- and middle-income countries. CT-based IGBT guidelines are rapidly evolving, which will provide more confidence in the practice of these hybrid approaches. Smaller sample size of our study cohort is a limitation that could create unintentional selection bias.