Introduction
Pancreatic ductal adenocarcinoma is currently the fourth leading cause of cancer deaths in Europe [1] and the third in the US [2]. At diagnosis, the disease is already metastatic in about a half of patients, while only 10–20% are eligible for resection. The remaining 30–40% are subdivided into borderline resectable pancreatic cancer (BRPC) and locally advanced pancreatic cancer (LAPC), based on the infiltration of large vessels. According to National Comprehensive Cancer Network® (NCCN) Guidelines [3], locally advanced tumour is defined as having any contact with the aorta, involving > 180° of large arteries (celiac axis or superior mesenteric artery) or involving large veins (portal or superior mesenteric vein) with no possible reconstruction. Other criteria exclude the potential resection with a lesser extent of involvement [4–6] (Table S1). In contrast to borderline resectable pancreatic cancer, the odds of radical resection after induction treatment in the LAPC group are generally limited. In retrospective studies the resection rates range 0–44% while prospective studies usually report the range of 4–12% [7–12].
Chemotherapy (CTx) regimens such as FOLFIRINOX (FFX) or gemcitabine plus nab-paclitaxel are sequentially administered alongside radiation therapy (RTx) or chemoradiation for the treatment of LAPC. Despite data indicating the improvement of quality of life (QoL) on FFX, the concerns related to its high toxicity led to the introduction of multiple modifications FOLFIRINOX (mFFX). Progressive disease is observed within the first 2–3 months of CTx in about 25–30% of patients, who are unlikely to benefit from subsequent radiotherapy [13]. Stereotactic body radiation therapy (SBRT) administers high doses of radiation onto the tumour area with very sharp dose gradient outside the target. In comparison to conventional RTx, the shorter course reduces the period of CTx, while faster delivery of biologically effective doses may provide better pain management and improvement in QoL. Additionally, SBRT has a favourable safety profile [14]. For these reasons, SBRT becomes an enticing option, with its usage rising 0.2–7.4% between 1998 and 2012 [15]. In addition to the increasing frequency of SBRT use in relation to pancreatic tumours in recent years, significant progress has also been noted in the technique itself of conducting SBRT. Currently, the most notable advancement is stereotactic magnetic resonance (MR)-guided adaptive radiotherapy (SMART) [16, 17]. SMART delivered with an MR system in comparison to cone-beam computed tomography (CBCT)-guided SBRT demonstrates superiority due to the utilisation of higher soft tissue contrast from MR imaging obtained before and during treatment delivery, automatic cessation of treatment in case of tumour displacement from its correct position, and daily adaptation of treatment plans on the table to account for anatomical changes between fractions [16]. In LAPC patients, SBRT is usually combined sequentially with systemic treatment, with either gemcitabine or modern regimens [9, 11, 18, 19] (FFX, mFFX, gemcitabine + nab-paclitaxel). Overall, such combinations seem relatively safe [11, 20] with the toxicity mostly pertaining to CTx regimens.
Herein, we report the results of a prospective study evaluating induction CTx with mFFX and subsequent SBRT in patients with locally advanced pancreatic cancer. We aimed to determine whether such a combination offers satisfactory efficacy, while preserving low toxicity and good QoL.
Material and methods
General study design and endpoints
This single-arm, single-centre, phase 2 study was conducted at Medical University of Silesia between January 2017 and December 2019. The study was approved by Bioethical Committee of Medical University of Silesia and registered at ClinicalTrial.gov, number NCT03891472. The study included adult patients with newly diagnosed (untreated), locally advanced pancreatic adenocarcinoma (Table S1) with otherwise good vital status (Eastern Cooperative Oncology Group performance status of 0–1) and no significant comorbidities (the detailed inclusion and exclusion criteria are listed in Table S2). Each patient was presented at an interdisciplinary tumour board, which included specialists in medical oncology, surgery, radiation oncology, and radiology. The primary endpoints were the resection rate and one-year overall survival (OS), defined as the time between the diagnosis and death or last follow-up. The secondary endpoints were progression-free survival (PFS), defined as time between therapy initiation and progression or death, toxi-city, and QoL. The CONSORT checklist is available in Table S3.
Treatment protocol
The patients were treated with 6 cycles of modified FOLFIRINOX every 2 weeks. The modification included a 25% reduction of irinotecan dose and 5-FU bolus omission (dosing as follows: oxaliplatin 85 mg/m2, levofolinic acid 200 mg/m2, irinotecan 135 mg/m2, 5-FU 1200 mg/m2) [21, 22]. During the sixth cycle of treatment, the response was evaluated by computed tomography (CT) according to response evaluation criteria in solid tumours 1.1 (RECIST) [23]. After progression exclusion, SBRT was administered in 5 fractions of 7 Gy every other day. Within 2 weeks of SBRT completion, the mFFX treatment was restarted and one to three cycles were administered. Eight (±1) weeks after SBRT the response was evaluated as above, and patients were qualified for surgical treatment. The definition of disease progression included radiological progression documented by imaging, clinical progression manifested by irreversible symptomatic deterioration, and disease progression (detection of cancer spread) confirmed intraoperatively and validated by histopathological examination. The patients were requested to complete the quality- of-life questionnaires (QLQ)-C30 and QLQ-PAN26 [24, 25] before treatment initiation on the first day and then every 2 weeks until surgery or treatment discontinuation. Toxicity was recorded with National Cancer Institute common terminology criteria for adverse events, version 4.0 [26]. The treatment protocol is depicted in Figure 1.
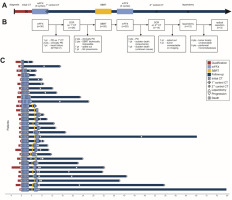
Fig. 1
Treatment regimen. Schematic diagram of the treatment regimen (A), study flowchart (B), course of treatment at the patient level (C)
CT – computed tomography, DCR – disease control rate, mFFX – modified FOLFIRINOX chemotherapy, NYHA – New York Heart Association , PD – progressive disease, SBRT – stereotactic body radiotherapy
Stereotactic body radiation therapy
Two to six gold fiducials were implanted percutaneously into or close to the tumour mass under ultrasound or computed tomography guidance. After 5 to 7 days, CT in the therapeutic position with oral and intravenous contrast was performed; breathing-related motion of the tumour mass was controlled using 4D CT. Abdominal compression was applied if the motions exceeded 5 mm. If the pancreatic tumour mass was not sufficiently imagined by CT, MR imaging was performed and fused in the radiotherapy planning system with the planning CT. Gross tumour volume (GTV) was defined using planning CT (arterial phase) together with fused MR imaging and positron emission tomography CT in some cases. 4D CT was used to define internal target volume (ITV), which was the sum of GTVs in different phases of the breathing cycle. The planning target volume (PTV) was obtained by adding a 3-mm margin to ITV. If ITV was close (< 3 mm) to the digestive tract, the PTV margin was individually adjusted to minimise the dose to critical organs at risk (OARs). The dose of 35 Gy, specified at the 80% isodose was delivered in five 7-Gy fractions every other day. In the case of close tumour mass contact or infiltration of the digestive tract, the sub-volume was defined with the total dose reduced to 25–30 Gy in five 5–6-Gy fractions in order not to exceed the OARs tolerance limits. Radiotherapy was delivered using Electa’s linear accelerators with volumetric modulated arc therapy technology. At least 95% of PTV was covered with specified dose and at least 99% of PTV was covered with 90% of the specified dose. The individually defined OARs were duodenal wall, gastric wall, intestinal wall, spinal cord, liver, and kidneys. The critical dose limits were specified according to institutional protocol. Before each fraction, cone-beam CT was used and adjustments in patient’s position were made according to the implanted gold fiducials or bony structures.
Statistical analysis
Statistical analysis was performed using R version 4.0.5 [27]. The required sample size was calculated using the clinfun package [28]. For the primary endpoints, the null hypotheses were radical resection rate of 10% [29, 30] (alternative: resection rate ≥ 30%) and one-year OS of 40% [31] (alternative: one-year OS ≥ 60%). With an enrolment of 30 patients, the null hypotheses would be rejected with at least 6 resections (α = 0.05, power = 0.84) and at least 16 patients surviving one year (α = 0.05, power = 0.72). No formal statistical testing was planned for secondary endpoints. The change in quality of life over time was analysed using maximum likelihood linear mixed-effects regression and type III analysis of variance with post hoc Student’s t-tests (vs. baseline) using Satterthwaite’s method [32, 33]; multiple comparisons were corrected with the Benjamini- Hochberg method. Subgroup analyses were precluded by insufficient power. For data visualisation the ggplot2 [34] and survminer [35] packages were used.
Results
Treatment outcomes
Thirty patients were enrolled and treated between January 2017 and January 2019. The patients ages ranged 42–77 years (median: 61); the M:F ratio was 2.0 (Table 1). Tumour unresectability was determined by infiltration of > 180° of arterial vessels in 29 patients (96.7%) and by unreconstructible involvement of venous vessels in 27 patients (90%); in 26 (86.7%) both criteria were fulfilled (Table 2).
Table 1
Study group characteristics
Table 2
Tumour-vascular ratio (expressed as an angle measure)
The first mFFX course (with the first control CT during the last cycle) was completed by 27 patients. The treatment was terminated due to early disease progression in 2 patients and aggravation of pre-existent chronic heart failure in one patient. Partial response or stable disease was observed in 26 patients (Table 3). Among these, SBRT could be initiated for 20 patients, all of whom received the complete planned dose and underwent the second mFFX course. The median time from the completion of first mFFX course to SBRT start was 47 days (range: 16–83 days). Due to the protocol defined period of 8 ±1 weeks between SBRT and the second radiologic evaluation, 2 patients received a single cycle, while 2 and 3 mFFX cycles were completed by 9 patients each. The second evaluation included 18 patients, with local control achieved in all patients; no signs of distant disease progression were detected in 14 patients (Table 3), who were subsequently referred for surgical treatment. The treatment course for each patient is depicted in Figure 1.
Table 3
Results of the first and second evaluation of response
Laparotomy was performed in 12 patients (one patient declined further treatment, and the tumour was locally advanced on imaging in the other). The median time from treatment initiation to surgery was 208 days (range: 176– 248 days). Radical resection was possible in 3 patients, which was less than the 6 assumed for the primary endpoint. The resection was abandoned due to local advancement in 4 patients and due to intraoperatively revealed peritoneal micrometastases (below the CT resolution) in 5 patients.
The analysis of CA 19-9 was also conducted: at baseline, after induction CTx, and before surgery. At baseline, CA 19-9 displayed a broad range, spanning 0.6–11526 U/ml (median value of 168.8 U/ml). Following the 6 mFFX cycles, CA 19-9 levels ranged 0.6–1740 U/ml (median value 87.1 U/ml). Among the 12 patients who underwent laparotomy, 3 consistently had CA 19-9 levels within the normal range, while the other 9 (with initially elevated values) demonstrated at least one instance of CA 19-9 decrease following treatment. Radical surgery was successfully performed in two patients whose CA 19-9 levels remained within the normal range throughout the entire treatment period and in one patient who achieved a decrease after mFFX, followed by a further reduction after SBRT.
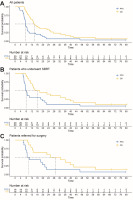
Sixteen patients (53.3%) were treated with second-line CTx, including FFX (n = 5), gemcitabine (n = 8), gemcitabine + nab-paclitaxel (n = 2), and De Gramont regimen (n = 1). The median PFS was 7.53 months (95% CI: 6.9–9.3) for the whole group (n = 30), 7.97 months (95% Cl: 7.2–19.57) for the SBRT group (n = 20), and 9.45 months (95% CI: 8.27–46.67) for the patients referred for surgery (n = 14) (Fig. 2). The median OS was 13.87 months (95% CI: 11.37–25.53) for the whole group (n = 30), 16.02 months (95% CI: 12.33–39.67) for the SBRT group (n = 20), and 29.12 months (95% CI: 12.97 – not reached) for the patients referred for surgery (n = 14) (Fig. 2). The one-year OS rate was 63.3% (19 patients), which was more than assumed for the primary endpoint. The median follow-up time was 73.98 months (95% CI: 68.23 – not reached). The 3-year OS rate was 16.67% (5 patients). Among those who experienced a disease recurrence/progression (n = 24), 41.67% had distant metastases as their first site of recurrence, 33.3% had both distant metastases and local progression, while 25% exhibited local progression.
Toxicity
Adverse events of all grades were assessed at each stage of treatment. Mild events were relatively common, while serious adverse events occurred in 8 patients during the whole treatment (the incidence of grade ≥ 3 events is reported in Table 4). Ten patients required dose delay (a single cycle delay for 9 patients, and 2 cycles were delayed in one patient); dose delay was required during the first and second course in 5 patients each. Dose reduction was necessary in 5 patients; the first dose of irinotecan was reduced in 4 patients (during a single cycle in 2 patients, and during 3 and 6 cycles in one patient each), while the first dose of irinotecan, oxaliplatin, and fluorouracil was reduced during one cycle in one patient. At the mFFX stage after SBRT, 2 deaths were recorded: one case of death secondary to severe pneumonia and one case of sudden death with unknown cause. All grade ≥ 2 early adverse events within 90 days from SBRT completion are reported in Table 5.
Table 4
Adverse events grade ≥ 3 which occurred during each stage of treatment
Table 5
Early adverse events (grade ≥ 2) which developed within 90 days from stereotactic body radiotherapy completion
Quality of life
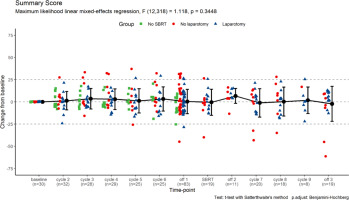
In total, 347 completed QoL questionnaires were returned. Each patient completed between 2 and 17 (median: 13) questionnaires, including the baseline. Due to a technical error, 3 questions were missing from the questionnaire forms handed to the patients (nos. 25, 29, and 30 of QLQ-C30) and are unevaluable. In general, we noted no consistent change in the overall quality of life reflected by the QLQ-C30 Summary Score (Fig. 3).
Fig. 3
The change of quality-of-life questionnaires C30 summary score from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error-bars; numbers of questionnaires returned for each time-point are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off-treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy
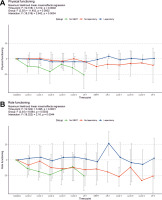
Regarding the functional scales, we noted some deterioration in physical and role functioning, but improvement in Emotional (p = 0.003) (Fig. 4 A) and Social Functioning (p = 0.004) (Fig. 4 B). On the other hand, some deterioration may be noted in both Physical (p = 0.030) (Fig. 5 A) and Role Functioning (p = 0.032) (Fig. 5 B) during the treatment course, which may reflect either tumour progression or side effects of the treatment. Therefore, we performed mixed-effects regression analysis including the subgrouping according to the ability to complete the protocol, which showed a significant association between both scales and the subgroups (Fig. 6).
Fig. 4
The change of quality-of-life questionnaires C30 emotional functioning (A) and social functioning (B) scales from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error bars; numbers of questionnaires returned for each timepoint are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off-treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy

Fig. 5
The change of quality-of-life questionnaires C30 physical functioning (A) and role functioning (B) scales from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error bars; numbers of questionnaires returned for each time-point are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy

Fig. 6
The change of quality-of-life questionnaires C30 physical functioning (A) and role functioning (B) scales from baseline levels. The means ± standard deviations for the subgroups are represented by error bars; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy
What is crucial, the patients consistently reported the alleviation of symptoms of pain (p < 0.001) (Fig. 7 A), pancreatic pain (p < 0.001) (Fig. 7 B), and insomnia (p = 0.005). We also observed an improvement of appetite (p = 0.026) (Fig. 8 A) in parallel with the aggravation of diarrhoea (p = 0.007) (Fig. 8 B). No exacerbation of fatigue, dyspnoea, cachexia, ascites, or hepatic-related symptoms (p > 0.05) were observed. We observed an increase in CTx-related side-effects (including dry mouth and altered taste) (Fig. 9 A). Additionally, a minor alleviation of the fear of future (Fig. 9 B) could be noted. Finally, there was no significant aggravation of financial difficulties or healthcare dissatisfaction (p > 0.05), with a minor deterioration in terms of body image and sexuality-related symptoms.
Fig. 7
The change of quality-of-life questionnaires (QLQ)-C30 pain (A) and QLQ-PAN26 pancreatic pain (B) scales from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error bars; numbers of questionnaires returned for each timepoint are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off-treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy

Fig. 8
The change of quality-of-life questionnaires C30 appetite loss (A) and diarrhoea (B) scales from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error bars; numbers of questionnaires returned for each time-point are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off-treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy

Fig. 9
The change of quality-of-life questionnaires PAN26 side effects (A) and fear of future (B) scales from baseline levels. The scores for individual patients at each time-point are represented by points (grouped according to treatment protocol completion); the means ± standard deviations for the whole group are represented by error bars; numbers of questionnaires returned for each time-point are also presented; statistical calculations were performed on non-transformed data. Time-point definitions: baseline – prior therapy initiation; cycle 2–6 – during the initial course of modified FOLFIRINOX chemotherapy (mFFX); off-1 – off-treatment period after the initial mFFX course and before stereotactic body radiotherapy (SBRT); SBRT – during SBRT; off-2 – off-treatment period after SBRT and before the additional mFFX course; cycle 7–9 – during the additional mFFX course; off-3 – off-treatment period after the additional mFFX course
SBRT – stereotactic body radiotherapy

Discussion
Pancreatic cancer remains an unrelenting challenge for contemporary oncology and is expected to become the second cause of cancer-related mortality within the next 2 decades [36]. It results from its increasing incidence, diagnosis typically at locally advanced or metastatic stage, as well as resistance to targeted therapies. Current investigations are particularly focused either on resectable or metastatic disease, while any progress in LAPC treatment is usually their derivative.
The trial was negative in terms of improving the resection rate in this setting. Nonetheless, it should not lead to the conclusion of a lack of benefit from the combination of mFFX and SBRT in LAPC patients. Following the induction therapy, 3 tumours were radically resected and 5 others responded sufficiently to become resectable, but previously unknown peritoneal micrometastases made the resection pointless. The lack of staging laparoscopy during recruitment is an undeniable shortcoming because metastatic disease may have been identified earlier. Secondly, our cohort included patients with extensive disease and involvement of multiple large vessels, both arterial and venous. The radical resection was successful in 2 out of 4 patients with venous or arterial involvement alone. A future study with stricter inclusion criteria may provide more conclusive evidence. Moreover, the mFFX/SBRT combination was associated with good disease control rates and minimal toxicity. We also monitored the potential decline of the on-treatment QoL due to multimodal therapy. Conversely, no deterioration in the global QoL was noted, while some symptoms (pain in particular) subsided. To date, the results of only a few prospective clinical trials evaluating the FFX/SBRT induction combination for LAPC have been published. In the LAPC-1 trial FFX was given in up to 8 cycles. After the completion of CTx and exclusion of disease progression the patients received SBRT at dose of 40 Gy in 5 fractions. In contrast to our research, their protocol included staging laparoscopy, which identified previously unknown metastases in 18/72 patients [11]. Fifty patients started the regimen and 39 underwent SBRT (78% vs. 67% in our study). Subsequently, radical resection was possible in 6 patients (12% of all included vs. 10% in our study), while progressive disease was identified on radiological examination in 27/39 patients (69% vs. 20% in our study). Both PFS (9 vs. 7.5 months) and OS (15 vs. 13.9 months) were slightly better than in our study. A probable bias of the LAPC-1 trial in patient selection was the LAPC definition according to Dutch pancreatic cancer criteria, where a tumour is defined as unresectable with a lesser vessel involvement than that described by NCCN Guidelines, which were employed in our study. In terms of toxicity, there were 34 grade ≥ 3 adverse events per 50 patients (vs. 11 events per 30 patients); in both studies toxicity occurred mostly during CTx. The quality of life cannot be compared because it was not assessed in the study by Suker et al. [11]. Overall, the efficacy of both regimens seems similar, while our modification provided a slightly better safety profile. The results of the long-term follow-up of the LAPC-1 trial were recently published [37]. In the whole population, the 1- and three-year OS were 62% and 10%, respectively, and were comparable to our results (63.3% and 16.67%). In the second prospective study, the patients received multi-agent CTx for a minimum of 3 months (65% of patients received FFX). After excluding disease progression, they underwent SBRT with a dose of 25–33 Gy delivered in 5 fractions [19]. Survival outcomes were better than in our study, the median OS was 21.6 months from diagnosis and 14.6 months from SBRT. Moreover, 38.6% of patients were surgically explored (40% in our study) and 94% of them were successfully resected (25% of these in our study). Even though both studies employed the NCCN criteria, such differences may potentially be attributed to variations in the degree of vessel involvement. It should be noted that in our study in almost patients multiple vessels were involved, with both arterial and venous vessels affected in 86.7%. In terms of toxicity, the comparison between the studies has limited value due to the lack of data on CTx-related toxicity. Regarding the assessment of QoL conducted in both studies using QLQ-C30 and QLQ-PAN26 questionnaires, no significant deterioration was observed either after CTx or after SBRT in either study [19]. In addition, a recent study with prospectively maintained database evaluated ablative radiotherapy of 98 Gy in LAPC patients (who had previously undergone multimodal CTx) with a low rate of adverse events [38]. The authors did not investigate the possibility of radical resection; however, the regimen was associated with promising outcomes (median OS of 18.4 months) and good locoregional control (cumulative 24-month locoregional failure rate of 32.8%). In the third prospective study, CTx with mFFX alone was compared to the combination of mFFX + SBRT. This phase III study (NCT01926197) has recently been completed, but the entire outcomes have not been published yet [39]. The limited provisional data posted on the clinicaltrials.gov server are difficult to interpret given the lack of baseline patient characteristics (other than age, sex, and ethnicity).
The second strategy employed in the treatment of LAPC is CTx alone without subsequent radiotherapy. To our knowledge, NEOLAP-AIO-PAK-0113 is the first trial to investigate the most active multidrug CTx regimens in a randomised controlled trial (RCT) of patients with LAPC [40]. In this open-label, multicentre, randomised phase 2 study, the patients after 2 cycles of nab-paclitaxel + gemcitabine without progressive disease were randomly assigned (1 : 1) to receive either 2 additional cycles of nab-paclitaxel plus gemcitabine (nab-paclitaxel plus gemcitabine group) or 4 cycles of sequential FOLFIRINOX (sequential FFX group). The primary endpoint was the surgical conversion rate in the randomised population by intention-to treat analysis, which was assessed by surgical exploration. A surgical conversion rate of 35.9% was obtained in the nab-paclitaxel plus gemcitabine group and 43.9% in the sequential FFX group [40]. Indeed, these results are significantly better than those in our study. Median OS was 18.5 months in the nab-paclitaxel plus gemcitabine group and 20.7 months in the sequential FFX group [40]. One of the reasons for the significantly improved outcomes in the NEOLAP- AIO-PAK-0113 study is the methodology employed for calculating resection rates and survival time. In total, 168 patients were enrolled in the study, with 165 undergoing treatment. Thirty-five patients who initially received 2 cycles of gemcitabine + nab-paclitaxel CTx were excluded from randomisation (primarily due to disease progression), while the resection rate and OS were calculated for the randomised cohort of 130 patients. In contrast, in our study, the analyses were conducted for all enrolled patients. The wider inclusion criteria also probably improved the outcomes. The NEOLAP-AIO-PAK-0113 study included patients with unresectable tumours based on the NCCN definitions as well as those with borderline resectable tumours and arterial involvement. It must be emphasised that the resection rates for BRPC following induction therapy with FFX exceed 50% [10]. In our study, BRPC was an exclusion criterion. In terms of toxicity, there were 35 grade ≥ 3 adverse events per 64 patients in the nab- paclitaxel plus gemcitabine group and 35 grade ≥ 3 adverse events per 66 patients in the sequential FFX group (vs. 11 events per 30 patients in our study). The quality of life cannot be compared because it was not assessed in the study by Kunzmann et al. [40].
One of the future challenges will be determining the management strategy for LAPC, especially whether exclusive systemic treatment or a combination with radiotherapy is optimal. The role of radiotherapy was analysed in a large study on 1835 patients who received mFFX as initial treatment for localised pancreatic adenocarcinoma, of whom 958 (52.2%) had LAPC [10]. Despite the lack of published RCT to support radiotherapy after mFFX, a total of 546 (57.7%) patients received subsequent radiotherapy in the LAPC group. In this study, patients who received additional radiotherapy following systemic treatment showed superior OS compared with those who did not (23.6 vs. 18.4 months; hazard ratio = 0.77, 95% CI: 0.69–0.87, p < 0.001). However, selection bias and guarantee-time bias may have influenced this comparison. The position of radiotherapy, including SBRT, remains unclear [10]. The complete results of the CONKO-007 trial may provide an answer regarding the role of radiotherapy in this setting [41]. To date, only the initial results were published in the form of a meeting abstract. The authors indicated that the addition of radiotherapy improves the R0 circumferential resection margin negative rate, which results in better outcomes. Nonetheless, this effect did not translate into a significant survival benefit in the whole cohort, which underlines the need for good predictive biomarkers [41].
When discussing the strategic role of radiotherapy in the management of locally advanced pancreatic adenocarcinoma, it is also important to highlight the continuous advancements in RTx techniques. The results of the first prospective phase 2 clinical trial on treating patients with LAPC using SMART radiation technique were recently published [16]. This study enrolled 136 participants, with 56.6% having LAPC and 43.4% BRPC. Eligible patients had received at least 3 months of any CTx without distant progression and had CA19-9 levels of 500 U/ml or lower. SMART treatment was administered using an MR-guided system, delivering a prescribed dose of 50 Gy over 5 fractions. Patients were allowed to undergo surgery and CTx after completing SMART. The primary endpoint of the study was the occurrence of late grade ≥ 3 gastrointestinal (GI) toxicity specifically attributed to SMART. The occurrence of acute and late grade ≥ 3 GI toxi-city, definitively related to SMART, was 0%. However, both acute and late toxicity associated with SMART have been recorded, designated as probably related or possibly related. This rate was similar to what has been reported following CBCT-guided SBRT [16, 35]. Since our study did not analyse late toxicity (> 90 days after SBRT administration), a comparison is not possible. The acute toxicity (< 90 days from SBRT) observed in our study appears to be comparable to the outcomes achieved with the use of SMART, although we did not define toxicity in our study as either definitely related or probably related or possibly related. Limitations of the SMART study include the enrolment of both BRPC and LAPC patients, the allowance of institutional resectability criteria instead of a standardised definition, and the permission of any induction CTx regimen. A key conclusion from this study is that in the context of utilising a higher biologically effective dose than in CBCT-guided SBRT, the use of a higher dose in SMART while maintaining safety suggests the superiority of this technique [16].
Several limitations of our study must be noted. Firstly, the number of included patients was low, and thus the study was prone to be affected by random effects. Secondly, during patient inclusion, we did not perform staging laparoscopy, which might have detected peritoneal metastases not visible on CT.
Conclusions
Finally, the vast majority of patients had extensive disease with the involvement of multiple large vessels, so the potential benefit from the induction therapy might have not been sufficient in this setting. However, it must be emphasised that strict adherence to NCCN criteria for distinction between borderline and locally advanced cancer is crucial because its lack may both harm the misclassified patients and obscure study results.
Taking into consideration the encouraging one- and three-year survival results in our study, while maintaining low treatment toxicity and preserving QoL simultaneously, the combination of mFFX and SBRT may serve as an alternative to treatment with CTx alone (associated with higher toxicity due to an increased exposure to cytostatics). Furthermore, in some patients we observed a decrease in CA 19-9 levels after SBRT despite the initial increase after mFFX. This might suggest that even among mFFX non-responders there are patients who benefit from SBRT. The biggest challenge is their accurate identification. To conclude, the combination of mFFX with SBRT merits further investigation in LAPC patients. In a variable proportion of patients, a subsequent radical resection is possible and should be pursued because it is known to improve survival. Currently, numerous clinical trials are ongoing in LAPC assessing the efficacy of multimodal systemic treatment in combination with SBRT (Table 6) [42–49]. Finally, early data suggest that gemcitabine + nab-paclitaxel may be a viable second-line option for patients treated with FFX [40], but the optimal therapeutic sequence must be determined with properly designed and adequately powered trials.
Table 6
Summary of ongoing trials in patients with locally advanced pancreatic cancer assessing the efficacy of multimodal systemic treatment in combination with stereotactic body radiotherapy
| Trial number | No. of patients | Phase | Stage | Therapy | Primary endpoint | Expected completion |
|---|---|---|---|---|---|---|
| NCT04986930 [42] | 92 | 2 | LAPC | mFFX mFFX + SBRT | PFS | 2024 |
| NCT04247165 [43] | 20 | 1/2 | LAPC | GnP + Nivo + Ipi + SBRT | AEs + SAEs rate | 2024 |
| NCT04089150 [44] | 120 | 2 | BRPC LAPC | GnP or mFFX GnP or mFFX + SBRT | LRR | 2025 |
| NCT02128100 [45] | 28 | 2 | LAPC | FFX + SBRT | AEs rate | 2025 |
| NCT04331041 [46] | 42 | 2 | BRPC LAPC | Chemo + SBRT Chemo + SBRT + Defactinib | PFS | 2025 |
| NCT04570943 [47] | 103 | 2 | LAPC | GnP/mFFX + MR-SBRT | DCR at 4 month | 2026 |
| NCT04698915 [48] | 160 | 2 | BRPC LAPC | ED “GC4711” + SBRT Placebo + SBRT | OS | 2027 |
| NCT05585554 [49] | 267 | – | LAPC | Chemo Chemo + SBRT | OS | 2028 |
[i] AEs – adverse events, BRPC – borderline pancreatic cancer, Chemo – chemotherapy, DCR – disease control rate, ED – experimental drug, FFX – FOLFIRINOX, GnP – gemcitabine + nab-paclitaxel, Ipi – Ipilimumab, LAPC – locally advanced pancreatic cancer, LRR – locoregional response rate, mFFX – modified FOLFIRINOX, MR-SBRT – MR-guided SBRT, Nivo – Nivolumab, OS – overall survival, PFS – progression-free survival, SAEs – severe adverse events, SBRT – stereotactic body radiotherapy