Introduction
Primary cicatricial alopecia (PCA) encompasses a heterogeneous group of inflammatory diseases characterized by the replacement of hair follicle structures by fibrous tissue. Although PCA represents only about 7% of patients consulted in specialist hair clinics [1], its incidence rate has been increasing over the last few decades [2]. According to the working classification of the North American Hair Research Society, PCA is divided into four groups based on the prominent inflammatory infiltrate [3]. Discoid lupus erythematosus (DLE) and lichen planopilaris (LPP) are the most common causes of scarring alopecia with lymphocytic infiltrate [1, 4].
LPP clinically presents as patch alopecic areas with follicular plugs, perifollicular scaling along with perifollicular erythema [5]. Besides classic LPP, another important variant of the disease is frontal fibrosing alopecia (FFA), which occurs in postmenopausal women presenting with progressive band of alopecia of the frontal and temporal hairline [4]. The trichoscopy of LPP displays several characteristic features such as the absence of follicular openings, peripilar casts and scaling, perifollicular erythema and white cicatricial areas [6]. Twenty percent of LPP patients present with classic violaceus papules of lichen planus in other areas of the body [7] and in less than 10 present we can observe mucosal involvement of the disease [8].
Lesions in the course of DLE on the scalp are often multiple and are more prominent on the vertex [9]. Morphological features to distinguish DLE from other mimickers include: erythematous hue due to the presence of arborising vessels, atrophic scarring and dyspigmentation along with follicular plugging [9]. In contrast to LPP, the signs of DLE activity are presented in the centre of the alopecic lesion [10]. DLE patients are at higher risk of developing systemic lupus erythematosus [10] as well as squamous cell carcinoma in the affected scalp areas [11].
In histopathology, interface changes are seen both in LPP (lichenoid) and DLE (vacuolar) lesional skin. In LPP, the perifollicular lymphocytic infiltrate is prominent in the superficial dermis while in DLE, the infiltrate is perifollicular and perivascular. Mucin deposition is more characteristic for DLE [12].
The distinction between both entities is often challenging because of significant clinical and histopathological overlap [13]. Moreover, different subtypes of the disease follow a common pathway of late-stage fibrosis in which histopathological findings become indistinguishable [14]. Early detection of the disease with prominent inflammation facilitates definitive diagnosis. Implementation of the proper therapy is crucial as the conditions follow a stepwise progression leading to permanent hair loss which can be damaging for the affected individual’s self-esteem [15]. These concerns underscore the need for useful additional tools to help make a diagnostic distinction between LPP and LE in everyday practice.
Although the pathogenesis of PCA remains not fully understood, the key to its understanding might be the location of the peri-follicular inflammatory infiltrate, which in PCA is centred around the bulge region [16]. This specific location exhibits the so-called immune privilege resulting from complex immunosuppressive mechanisms, such as suppression of dendritic cells (DCs) activity [17, 18]. DCs are divided into five major subsets: plasmacytoid DCs (pDCs), myeloid/conventional DC1 (cDC1), myeloid/conventional DC2 (cDC2), Langerhans cells (LCs) and monocyte-derived dendritic cells (moDCs) [19]. Initiation and control of immune responses require a wide range of mechanisms and responses. LCs take part in tolerance maintenance to commensals as well as in responses to selected pathogens. pDCs are natural type I-interferon (IFN type I) producers. While cDC1 and cDC2 play an important role in antigen processing and presentation, Mo-DCs are active mainly at the site of an active disease and produce inflammatory cytokines [19].
Aim
It has been shown by prior work of others [13, 20, 21] that clusters of pDCs might help distinguish DLE from LPP. We hypothesized that other subsets of dendritic cells can provide more thorough and reliable histopathological clue to distinguish between these two entities.
Material and methods
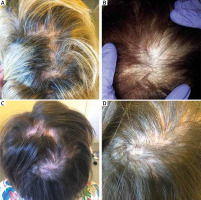
In this retrospective cohort study 51 patients diagnosed with LPP and DLE were recruited from the Department of Dermatology at the University Hospital in Krakow in accordance with an approved Institutional Review Board protocol (Table 1). All subjects provided written informed consent. All patients were grouped accordingly to two groups: LPP and DLE patients based on the histopathological and clinical evaluation (Figure 1).
Table 1
Epidemiologic characteristics of the study population
| Characteristics | LPP (n = 33) | DLE (n = 18) |
|---|---|---|
| Age, mean ± SEM | 57.23 ±10.39 | 58.17 ±12.86 |
| Sex, n (%): | ||
| Female | 32 (97.0) | 17 (94.4) |
| Male | 1 (3.0) | 1 (5.6) |
Skin biopsies had been taken before the treatment was started. Cell count was performed on immunohistochemistry by using characteristic monoclonal antibodies to specific subpopulations of DCs: CD1a and langerin (CD207) (LCs) CD11c (cDC2), CD206 (moDCs) and CD123 (pDCs) (LEICA Biosystems). Cutaneous tissue samples were stained manually and processed according to the protocol used on a routine basis in the laboratory of the Department of Pathomorphology in Krakow. The selected paraffin-embedded tissue blocks were cut into 4 µm thick sections, mounted on SuperFrost glass slides (ThermoScientific, USA) and dried in an incubator for 12 h in 34°C. The obtained slides were deparaffinised, dehydrated and then incubated in 3% H2O2 solution for 10 min to block endogenous peroxidase activity. Antigen retrieval was performed by immersing the slides in citrate buffer (pH 6.0; 0.01 M) or EDTA (pH 8.0; 0.01 M) and subjecting them to 97°C in a water bath for 30 min. Polyclonal secondary antibodies conjugated to horseradish peroxidase (HRP) enzyme (Ultra Vision LP Value Detection System HRP Polymer, Lab Vision, ThermoScientific, USA), were applied to visualize the obtained antigen-antibody complexes, using DAB (3,3’-diaminobenzidine) as chromogen. Cell nuclei were stained with haematoxylin to enhance contrast in tissue sections.
Evaluation of immunostaining
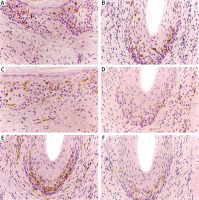
Quantitative assessment of each DC subset was performed in light microscopy on the basis of the numbers of positively stained cells (membrane and cytoplasmic brown staining). The number of positively stained cells was counted at 40× magnification and expressed per high power field. The number of dendritic cells was evaluated in hair epithelium of affected follicle and within the area of perifollicular fibrosis separately, for monoclonal antibodies CD123, CD11c and CD206 independently and combined for CD1a and langerin in every cutaneous sample (Figures 2 B, D–F). The dendritic cells were also counted in epidermis beyond the follicular ostia and in dermis beyond the hair follicles separately, at 40× magnification and expressed per high power field (Figure 2 A, C).
Figure 2
Immunohistochemistry. A – CD1a/CD207+ cells in the epidermis in LPP. B – CD1a/CD207+ cells within the hair follicle in DLE. C – CD1a/CD207+ cells in the dermis in DLE. D – Intra- and peri-follicular location of CD206+ cells in LPP. E – CD206+ cells within the hair follicle and within the area of perifollicular fibrosis in DLE. F – CD123+ cells within the hair follicle in DLE
Results
The distribution of CD123+ cells was similar in both PCA groups. The infiltrate composed of CD123+ cells was the smallest between all DC subsets and was localized mostly in the perifollicular area. Single CD123+ cells were found in the epidermis. A similar pattern of distribution was presented by CD11c+ cells without distinction between DLE and LPP groups. CD206+ cells were prominent in the perifollicular area as well as in the dermis in both groups. CD1a/CD207+ cells were commonly observed in the epidermis although in DLE patients they were also abundant within the hair follicle. In the bulge region of the hair follicle the infiltrate composed of DCs was smaller and we did not observe any differences between two analysed entities (Supplementary material).
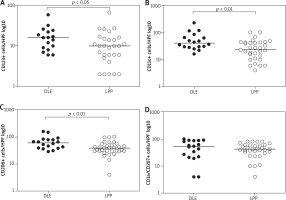
Patients with DLE were found to have significantly more CD123+ cells in their hair epithelium compared to those with LPP (median: 15 vs. 7) (p = 0.027) (Figure 3 A).
The inflammatory infiltrate composed of CD11c+ cells was also statistically higher in the DLE group in comparison to patients with LPP (median: 40.5 vs. 23) (p = 0.0054) (Figure 3 B).
Moreover, DLE patients were found to have significantly more CD206+ cells in their lesional skin compared to those who presented LPP lesions (median: 60.5 vs. 39) (p = 0.0059) (Figure 3 C).
Although the number of CD1a/langerin+ cells showed a tendency to be higher in the DLE group than the LPP group (median: 32.5 vs. 27), the difference was not statistically significant (p = 0.2366) (Figure 3 D).
Discussion
According to the working classification of the North American Hair Research Society, primary cicatricial alopecia is divided into four groups based on the prominent inflammatory infiltrate [3]. Unfortunately, the clinical and histopathological features of the most common conditions – DLE and LPP – often overlap and become indistinguishable. The study by Nambudiri et al. reinforces that correct distinction between these two entities presents a great diagnostic challenge for dermatologists and pathologists [22]. A proper diagnosis not only facilitates an effective therapy, but is also important in terms of monitoring the patients towards development of other conditions such as systemic lupus erythematosus. Since there is a need for an additional tool in diagnostic algorithm, in the presented study we report the differences in DC counts between DLE and LPP patients.
pDCs are the major type I interferon producers to pathogenic agents that play an important role in innate immunity [19]. pDCs are bone-marrow derived cells, initially localized in reactive lymphoid organs and are typically absent in healthy skin [19]. Their presence in the form of clusters have been already described as a highly predictive finding in DLE lesional skin [13, 20]. Fening et al. demonstrated that pDCs constituting more than 20% of the inflammatory infiltrate and pDCs clusters with more than 20 cells seem to exclude LPP and favour the diagnosis of DLE [20]. These findings are partially consistent with the results of the presented study in which we observed higher pDCs infiltrate in DLE patients in comparison with the LPP group although pDCs did not form clusters as their distribution was more diffuse in the perifollicular area without distinction to PCA diagnosis.
cDC2s represent the major subset of myeloid DCs in blood and they have the ability to stimulate naïve T cells. Myeloid cDC2s are equipped with a wide range of receptors such as lectins or toll-like receptors (TLRs) and produce inflammatory cytokines in response to TLRs stimulation. They also have the capacity to present the glycolipid antigens of mycobacteria and other pathogens [19]. Méndez-Reguera et al. found an increase in cDCs in the skin of DLE patients, compared with controls. Interestingly, cDCs from the DLE patients showed a high expression of CCR6, which correlated with disease activity [23]. We report for the first time the differences in cDC2s counts between two types of scarring alopecia. The role of myeloid DCs in the pathogenesis of DLE alopecia remains to be elucidated in the future studies.
So far, our study is also the first to show an increase in monocyte-derived DCs in lesional skin of DLE patients, compared with LPP. Their pattern of distribution was similar in both groups. moDCs recruitment and activation is accelerated in the inflammation. For this reason they are often called “inflammatory dendritic cells” [19]. moDCs present the ability to secrete various cytokines and may play a role in T-cell differentiation towards pathogenic lymphocytes Th17 implicated in lupus erythematosus pathogenesis [24].
Langerhans cells are specialized DCs found in basal and squamous layers of epidermis [19]. In inflammation, LCs lose their connections with the epithelial layer and migrate into the afferent lymphatics. Although LCs play an important role in maintaining epidermal health and tolerance to commensals, they can also present mycobacterial glycolipid and stimulate CD8 T cells [19]. Moresi and Horn mapped and quantified the distribution of LCs within follicular epithelium in normal human skin [25]. They reported infundibulocentric distribution of LCs which may correspond to the pattern of follicular inflammation in scarring alopecia. They hypothesized that LCs might act as a trigger or target in T-cell mediated processes in response to an unknown exogenous antigen. In the presented study the infiltrate comprised of LCs was higher in the skin of DLE patients, particularly within hair epithelium, compared to LPP ones but the difference was not statistically important.
The limitation of our study was inclusion of cases with sufficient clinicopathological correlation and exclusion of cases with late-stage fibrosis. Therefore, in the future studies we plan to validate our results in an independent cohort.
In the presented study we demonstrated that almost all subpopulations of dendritic cells were highly expressed in lesional skin of discoid lupus erythematosus patients in comparison with lichen planopilaris ones. In the light of this observation, dendritic cells might be used as an additional clue in differential diagnosis of PCA.