Introduction
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myositis presenting principally with heliotrope rush, Gottron’s papules and various dermatological manifestations including erythema and telangiectasia among others [1]. Polymyositis (PM) is an idiopathic inflammatory myopathy as well, characterized by symmetric muscle weakness and accompanied by a high skeletal muscle enzyme serum level and diagnosed through histopathological and electromyographical findings [2]. Myositis in general was related to malignancy as early as in 1916 by Sterz et al., who described it as a paraneoplastic syndrome. Paraneoplastic manifestations include a wide variety of diseases, mainly categorized in four large groups, consisting of endocrinological, hematological, neurological and dermatological symptoms, which fail to be attributed to effects of the primary tumor itself, its metastases or hormones produced by the tissues affected [3]. Although the type of malignancy varies with PM complicating mainly Hodgkin’s lymphoma while DM is usually associated with ovarian. breast, colorectal, lung, breast, pancreas, stomach and prostate. The mechanism behind this relationship seems to lie on the vicious circle of activation of the complement system resulting in endomuscular capillaries lysis and further inflammation due to muscle ischemia [4]. The incidence of DM is rather low affecting 0.5–0.89 per 100,000 patients, female predominating male with a ratio 2 : 1 [5]. DM and PM respectively are highly associated with the risk of cancer development, which may precede or coincide with the diagnosis or follow it in cases of paraneoplastic DM/PM. Breast, lung, colorectal and nasopharyngeal cancer have been among others mostly correlated with the presence of inflammatory myositis while PM specifically is related to Hodgkin lymphoma and other types of lymphoma [6]. Ovarian cancer and DM have been described to coexist [1]. Based on our findings, 110 similar cases have been reported in the literature. In a study by Cheng et al., the risk of developing myositis is low but its peak lies in an interval of 3 years before or after the diagnosis of ovarian cancer, since the disease can precede, coexist or follow the malignancy [1].
In this study, we conducted a systematic review of the literature aiming to present the relationship between ovarian cancer and DM or PM, with the ultimate purpose to set ovarian cancer among the underlying causes of idiopathic inflammatory myopathies and therefore suggest a diagnostic work-up for ovarian malignancy in DM or PM patients.
Material and methods
Data sources
We conducted a systematic search in PubMed and Scopus up to May 2020 using the key words: (ovarian cancer) and (dermatomyositis or polymyositis or myositis) as search terms. The search was performed by two independent authors VP and IDG. The inclusion criteria were well specified and no discrepancies in search results were found.
Study selection criteria
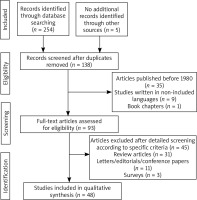
We reviewed all studies presenting a relationship between ovarian cancer and DM and PM. The inclusion criteria were: patients with ovarian cancer diagnosis that presented with DM and/or PM. The search string is described in Fig. 1. Articles published before 1980, studies written in languages other than English, German and Greek, book chapters, review articles, letters/editorials/conference papers as well as surveys and animal studies were not included in our study. Letters that did not mention any correlation between and ovarian cancer to DM or vice versa, have been excluded from our analysis. Furthermore, 45 articles were excluded after detailed screening according to specific criteria (Fig. 1). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed and carrying out at this systematic review [7].
Results
Selected studies
We retrieved a total of 135 studies. 45 articles were considered to be eligible for inclusion in our review, while 90 were excluded.
Table 1 summarizes the data collected from studies presenting cases with ovarian cancer coexisting with an inflammatory myopathy. Specifically, 48 scientific papers and 110 patients were studied [8–54]. The median age of the patients was 52.5 years (8–85). Regarding the oncological features of ovarian cancer, epithelial ovarian cancer (HG adenocarcinoma / serous / papillary / clear cell / endometrioid) was the major histological type of cancer in 79.1% of the cases (87/110). More specifically, serous carcinoma was diagnosed in 43.6% (48/110), papillary in 6.4% (7/110), while endometrioid in 3.6% (4/110) and clear cell carcinoma in 21.8% (2/110). Interestingly, the histology in 2 patients (1.8%) proved dysgerminoma and 3 (2.7%) women suffered from a poorly differentiated malignancy. Rarely, metastatic cancer was also found in 0.9% (1/110) and 1.8% (2/110) were diagnosed with mature teratoma, 0.9% (1/110) with transitional carcinoma, 0.9% (1/110) with signet ring carcinoma, 0.9% (1/110) with large cells carcinoma and 0.9% (1/110) with a malignancy of Mullerian origin. The FIGO stage of the disease at the time of the diagnosis was I in 2.7% (3/110), II in 4.6% (5/110), III in 59.1% (65/110) and IV in 18.2% (20/110) patients respectively.
Table 1
Main characteristics and outcomes of the patients with dermatomyositis and ovarian cancer
DM and PMf were the first manifestation of the disease in 63.6% (70/110) and 5.5% (6/110) respectively, while in 32.7% (36/110), ovarian cancer was already diagnosed and myositis was identified as a paraneoplastic syndrome. In 5.5% (6/110) other rheumatological (clinical amyopathic dermatomyositis (CADM), panniculitis, ichthyosis, poikiloderma, cutaneous Herpes zoster) conditions were also identified.
Regarding laboratory findings, median creatine kinase level was 886 (56.6–16,596) and serum antibodies were present in 22.5% (25/110) (ANA, anti-Jo1, anti-ds-DNA, anti-TIF1c, RNP, anti SS-A, anti-cardiolipin IgM, striational (Striated Muscle) antibody, P/Q-type calcium channel antibody, anti-p155/140, IgA and IgG).
The majority of the patients (70.9%, 78/110) were treated with primary surgery, while 4.6% (5/110) underwent interval debulking surgery. Twelve women (10.9% 12/110) were treated with chemotherapy, while in 0.9% (1/110) combined chemotherapy and radiotherapy was used. Three patients (2.7%, 3/110) did not undertake any treatment, one because of a deadly trauma. For 10% (11/110) of the patients, data about the treatment methods were not available.
In 24.6% (27/110) women postoperative recession of the symptoms was immediately observed, while 24.6% (27/110) patients too as well still needed rheumatological treatment after surgery. Specifically, prednisone was used in patients 8.9% (9/110), prednisolone in 4.6% (5/110), hydrocortisone in 0.9% (1/110) while steroid use in general is reported in patients 5.5% (6/110). Methotrexate was used in cases 4.6% (5/110) while azathioprine and intravenous immunoglobulin were used in cases 3.6% (4/110) respectively. Other medical factors reported include tacrolimus, fluocinonide and hydroxychloroquine, all used once (0.9%) in the so far published literature.
Neoadjuvant chemotherapy or radiotherapy was necessary in 58.2% (64/110) and a cancer recurrence was identified in 28.2% (31/110), with a median follow up of 24.5 months (5–210). Finally, 52 (47.3%) deaths were reported in a median follow-up period of 16 months (0–210).
Discussion
A systematic review was conducted in order to determine the association between DM and PM and ovarian cancer. According to our results, the diseases often coexist, and a full cancer screening should be considered for diagnosing ovarian cancer among patients with myositis. DM is very uncommon; so, a systematic review is a useful tool to evaluate the clinical and diagnostic characteristics of the disease as well as the epidemiological features of its association with ovarian cancer. Although the number of articles included in our study is not large, the number of patients that we examined is significant, compared to the epidemiologic data of DM and concomitant ovarian cancer in the literature [55].
Furthermore, it has been found that the prognosis in DM patients with malignancy is worse since typically the stage of the disease at the time of the diagnosis is already advanced [56].
DM presents with a wide range of symptoms including painless proximal symmetrical weakness as well as weakness of the pharyngeal muscle, often leading to aspiration pneumonia and dysphagia, conditions with the worst prognosis. Other clinical features encompass gastrointestinal symptoms and more rarely involvement of the ventilatory muscles. Nevertheless, pathognomonic of the disease are the skin lesions that are observed, that be Gottron papules and heliotrope rash [57]. Gkegkes et al. in their study about colorectal cancer and paraneoplastic DM demonstrate that the symptoms usually resolve after the successful treatment of the underlying malignancy [3].
Regarding the pathogenesis of the disease, many hypotheses have been suggested, mainly of autoimmune nature against regenerating myoblasts. The inflammatory reactions in the skin and muscles, are also escorted by inflammatory autoantigens expression mediated by neoplastic and muscle cells [3]. Especially, a high level of anti-p155/140 auto-antibody, should raise suspicion for undiagnosed ovarian cancer. Microangiopathy is also observed, with muscle capillary involvement, due to presence of auto-antibodies too. After all, the diagnosis is confirmed with muscle or skin biopsies. On the one hand, skin changes include vascular dermis-epidermis lesions, inflammatory lymphocytic infiltration and perivascular infiltration while in the muscled capillary obliteration and thrombosis combined with destruction of the endothelial cells as well as the perivascular and perifasicular tissues [17].
In an analysis by Cherin et al. total ovarian cancer incidence in DM patients was 13.3%, while in women aged over 40 years old, the incidence was as high as 21.4% [54]. Iavazzo et al. report of a case of PM presented following the successful treatment of a recurrent double primary breast and ovarian carcinoma [2]. After all, in a study by Field et al. it is strongly recommended that all patients with presenting with DM and breast or ovarian cancers should undergo genetic testing, since a plethora of such patients were found to carry BRCA mutations [44]. In the present study, in 76 women, the myositis was manifested first in comparison to 36 ovarian cancer patients, who were later diagnosed with paraneoplastic DM or PM. The diagnosis was based on the clinical features and the elevated serum creatinine kinase, which had a median level of 886 U/l as well as the presence of auto-antibodies, which were found positive in 25 women. Creatine kinase (CK) is a typical laboratory marker to measure the involvement of the muscles in DM. Interestingly, in the propensity analysis by Cheng et al. [1], a correlation was found between CK level and Ca-125 tumor marker level in almost 50% of the patients with concurrence of ovarian cancer and DM that were studied. Auto-antibody anti-Jo-1 is also frequently present, among other auto-antibodies such as anti-Mi-2 and antinuclear antibodies, the last one being though non-diagnostic for the disease [58].
Regarding histology, the most common histological type of the tumors was epithelial (79,1%) while rare cases of mature teratomas and dysgerminomas were also published [34, 37, 45, 48]. In fact, Roselino et al. [34] report a case of an 8-years-old child suffering from DM accompanied by ichthyosis which later was proven to be an underlying dysgerminoma, while a similar case of juvenile dysgerminoma and coexisting DM in a 16-year-old is described by Solomon and Maurer [37]. Except for the histology of the tumors included in our review, with an epidemiological distribution typical of the worldwide distribution of the disease, what was also in harmony with the data we found in the literature, was the advanced stage of malignancy, that most of our patients were diagnosed with. Specifically, Cheng et al. [1] in their analysis of 23 women with ovarian cancer and DM have found that 82.6% and 17.4% of them were diagnosed with FIGO stage III and IV respectively. In our study, stage II and IV were represented from 65 (59.1%) and 20 (18.2%) of the patients respectively, while stage I was diagnosed only in 3 (2.7%) and stage II only in 5 (4.6%) of the women included.
Despite the advanced stage, the majority of the patients 78 (70.9%) were managed by surgical treatment, followed by chemotherapy or radiotherapy in 64 of them (58.2%), while the rest of the patients were basically treated with chemotherapy, alone or with consequent interval debulking surgery. Whichever treatment, resolution of DM was observed at the same rate as in the cases where post-treatment, rheumatological factors, typically steroids, were needed (24.6% respectively). Tembe et al. in a study of 10 patients with paraneoplastic DM and PM, reports 3 cases with advanced stage ovarian adenocarcinoma, all treated with chemotherapy, who demanded post-treatment therapy with a combination of methotrexate, azathioprine and steroids [51].
The course of the patients studied varied in terms of survival and recurrence rated. In total, patients were followed for a period of 24.5 months and death is reported in 52 cases (47.3%). These findings are in agreement with those of a propensity score analysis by Chen et al., where overall survival as well as 5-year progression-free rates of patients with ovarian cancer and DM were poorer in comparison with ovarian cancer patients not suffering from DM (71.6% vs. 51.8%, p = 0.02 and 30.5% vs. 0%, p = 0.018 accordingly) [1]. However, an interesting case is reported by Girouard et al., where a 63-years old patient with metastatic ovarian cancer and DM-associated panniculitis, was diagnosed with 4 recurrences and was still alive after a 210-months follow up, the longest in the present study [18]. On the other hand, Davis et al. and Aydi et al. both report cases of immediate death after the cancer diagnoses [13, 35], the last one in a patient with rare histology of a signet ring cell carcinoma. In total, patients were followed for a period of 24.5 months and death is reported in 52 cases (47.3%).
Our results indicate that although rare, the prevalence of underlying ovarian cancer in patients with DM/PM is significant, so a thorough screening for an occult malignancy should be conducted, including in female patients an abdominal CT or MRI scan and serum levels of Ca-125 tumor marker, independent of the age of the patient. Recent clinical guidelines indicate additionally to Ca-125 levels, a pelvic examination and a transvaginal ultrasound every 6–12 months for 2–5 years after the diagnosis of DM is set [5]. Nevertheless, larger studies should be done in order to form universal guidelines with regards to the cancer screening in DM/PM patients.
To our knowledge, this is the first systematic review in the literature regarding the association of DM and PM and ovarian neoplasia. A detailed search of the published studies by two independent authors aimed in the elimination of the data loss. However, a number of limitations should be taken under consideration, before drawing conclusions. First of all, the number of the patients included is small, as a result of the limited number of relevant studies published. Furthermore, many studies were written in languages other than English and thus excluded from our review, while the included studies were all retrospective and individual presentation of random cases was prevalent. Another limitation is the fact that the diagnosis of DM is both rare and difficult, thus, restricting the cases presented in the literature in total. As a result, further, larger, well-designed studies are necessary to determine the optimal cancer screening for female patients with DM and/or PM.
Conclusions
DM and/or PM commonly relate to ovarian cancer, although the pathogenesis of both the disease itself and its association with ovarian malignancy is not completely understood yet. Hence, it is crucial that a full cancer screening including abdominal depictions and Ca-125 level measurements should be conducted in any DM/PM female patient, with a high suspicion for ovarian neoplasia. Moreover, in women with diagnosed ovarian carcinoma and emerging symptoms of DM/PM, a thorough check for a cancer recurrence is needed. Regarding the treatment of DM, cancer treatment should be attempted first, since in the majority of the cases, the symptoms resolve consequently.