Purpose
Image-guided adaptive brachytherapy (IGABT) is crucial for the treatment of advanced gynecological cancers [1,2,3,4,5]. The benefits of the combined intracavitary/interstitial (IC/IS) approach in advanced cervical cancer brachytherapy (BT) have already been demonstrated [6,7,8,9,10,11]. Standard applicators, such as Vienna-type ring applicator or Utrecht-type ovoid applicator, do not enable an adequate coverage of the target volume in all patients with advanced cervical cancer [3,12,13]. The 3D printing technology show promising results in different aspects of medical science and also in radiation oncology [14,15,16,17,18,19,20,21,22]. Patient specific boluses for radiotherapy can be designed for irregular surfaces to improve dose distribution. It is an attractive method for in-hospital design and modeling of BT applicators for skin, head and neck, breast, prostate, and gynecological cancers. Complex implantations can be performed, and accurate dose delivery can be achieved with individual template-guided procedures. This is of special importance in head and neck cancer due to the proximity of organs at risk (OARs). The process of manufacturing customized applicators is fast and financially viable. 3D printing technologies commonly used in medicine are stereolithography (SLA) and selective laser sintering (SLS). Different magnetic resonance imaging (MRI) compatible materials, in the form of a powder, liquid resin, or photopolymers, registered for use in medicine, are available and can be applied in BT. The material used determines the applicator’s properties. The final product needs to be firm and have a smooth surface.
The most important aspects of 3D-printed applicators are suitability for patient’s anatomy, better needle guidance, improved target coverage, and less trauma to the surrounding tissue and OARs. Consequently, smaller dose applied to OARs and reduced toxicity are expected. A better target coverage can be translated in higher tumor control probability [4,13,23,24,25,26,27].
We started using 3D printing technology for individual BT applications in 2015 [20]. In this paper, we present the results of a single-institution, one arm, non-randomized feasibility study on 3D-printed applicators for individualized gynecological cancer BT.
Material and methods
Nine patients were included in the study, and they were treated with external beam radiotherapy (EBRT) using volumetric modulated arc therapy (VMAT) or intensity modulated radiation therapy (IMRT) to 45-50 Gy prescribed in 25-28 fractions. Patients and tumor characteristics are presented in Table 1. Patients received concomitant cisplatin-based chemotherapy, 40 mg/m2 once per week in case of cervical or vaginal cancer. Carboplatin was used in case of renal dysfunction. EBRT was followed by two BT fractions. An additional BT insertion with standard IC applicator, usually in paracervical anesthesia, was performed in six patients with large-volume tumors or a narrow vagina one week before first BT treatment for virtual planning. In the remaining three patients, MR images from the first BT treatment with the standard IC/IS applicator in place were used for virtual planning if the dose parameters from the plan were suboptimal.
Table 1
Patients and tumor characteristics
| Characteristics | n |
|---|---|
| Age (years), median (range) | 59 (28-75) |
| Tumor type | |
| Cervical cancer | 7 |
| Endometrial cancer | 1 |
| Vaginal cancer | 1 |
| Histology | |
| Squamous cell carcinoma | 7 |
| Adenocarcinoma | 2 |
| Stage | |
| Cervical cancer stage IIIb* | 5 |
| Recurrent cervical cancer | 2 |
| Recurrent endometrial cancer | 1 |
| Vaginal cancer stage IVa* | 1 |
| Chemotherapy | |
| Cisplatin 40 mg/m2 (≥ 2 cycles) | 5 |
| Carboplatin AUC 1.5 (5 cycles) | 1 |
| None | 3 |
Patients were treated with PDR GammaMed Plus iX afterloader (Varian Medical Systems). The dose aim to the target volume D90 high-risk clinical target volume (CTV-THR) was 15.0 Gy in 22 pulses for vaginal cancer and 18.5 Gy in 25 pulses for cervical or recurrent endometrial cancer, with usually one week between fractions. Imaging with the applicator in situ was performed with GE Optima MR450w 1.5T MRI scanner. T2-weighted fast spin-echo sequence with a voxel size of 0.6 × 0.6 × 3.6 mm, and 3D isotropic T2-weighted fast spin-echo sequence with a voxel size of 1.0 × 1.0 × 1.0 mm were used. Contouring and treatment planning were performed in the BrachyVision treatment planning system (Varian Medical Systems).
Patient selection
Patients with locally advanced primary or recurrent cervical, endometrial, and vaginal cancer were eligible for the study. In the process of patients’ selection for individualized BT, several factors were taken into consideration. The most important of these was the extent of primary tumor (parametrial or paravaginal extension, involvement of sacrouterine ligament, pararectal space, or adjacent organs). Other factors were anatomical characteristics such as narrow vagina not suitable for standard applicator insertion, or the proximity of blood vessels. The following factors obtained from the plan using a standard BT applicator were also considered as an indication for the use of individualized applicator: exceeded dose to OARs and suboptimal dose-volume parameters for CTV-THR (V100 ≤ 90% of the CTV-THR volume and D98 ≤ 80%, D90 ≤ 100%, and D100 ≤ 60% of the dose aim to D90 CTV-THR).
Virtual plan and modelling of individual applicators
Plan A was a virtual plan and the starting point for 3D individual applicator construction. It was based on MRI from the first BT application, with the standard applicator in situ. The standard applicator for primary cervical and vaginal cancer of the upper part of vagina was a tandem and ring applicator, with a cap add-on for parallel interstitial needles (Vienna-type ring applicator). In patients after hysterectomy, a ring with a cap for parallel needles or a vaginal cylinder was used as the standard applicator [28]. CTV-THR, gross tumor volume (GTV-T), and OARs were delineated on MRI. The same concept for the target volume definition and delineation as for cervical cancer was transferred to vaginal and recurrent endometrial or recurrent cervical cancer. CTV-THR was defined as palpable mass at the time of BT and all grey zones visible on T2-weighted MRI. GTV-T was included in CTV-THR and was defined as macroscopic tumor (visible and/or palpable mass) at the time of BT and high signal regions on T2-weighted MRI.
Parallel and oblique needle positions were adapted to the tumor volume. The exact needle entry point location, angle, and depth of insertion into the tissue were defined for each needle. The angle of oblique needle insertion was defined as the angle between the tandem axis and the needle axis, or in patients after hysterectomy as the angle between the ring or vaginal cylinder central axis and the needle axis. The aim of plan A was optimal CTV-THR coverage. In this step, the dose to OARs was allowed to exceed the dose constraints due to OARs inter-fraction variability. In the next step, standard applicator model was individually adapted by adding needle guiding holes according to plan A.
Several types of individual applicators were designed. In 5 patients with cervical cancer, a tandem plus ring with a 3D-printed add-on for parallel and oblique needles was used. In 3 patients with recurrent cervical or endometrial cancer after hysterectomy, a ring with 3D-printed add-on was used. In one patient with vaginal cancer, a multichannel cylinder was used. Some solutions are presented in Figures 1 and 2. In narrow vagina, two applicator designs were proposed. The first design was a ring-shaped applicator, with twelve holes on the ring circumference for the parallel needles. Dwell positions in the needles at the level of the ring were used as a replacement of dwell positions in the active channel of Vienna-type ring applicator. The second design was a multi-channel vaginal cylinder (Figure 2).
Fig. 1
A) 3D-printed add-on for the ring with parallel and oblique needles. If 3D-printed cap add-on is used, the outside diameter of the ring is increased by 7 mm; B) 3D-printed tandem and ring. The size of the ring can be adjusted to the diameter of the vagina, and an oblique needle insertion at an angle of up to 45 degrees is possible; C) 3D-printed tandem with a needle inserted at a 15-degree angle
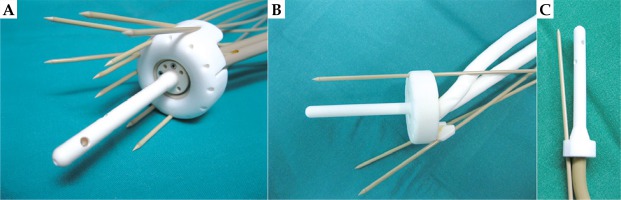
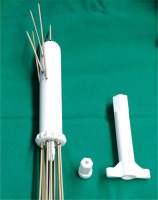
Fig. 2
3D-printed multi-channel vaginal cylinder with intracavitary/interstitial needles. Appropriate for narrow vagina and multiple vaginal lesions. A handle (right) is used to tighten the nut (middle) on the collect chuck of the cylinder for needle fixation. It is used at the end of application to fix all the needles at the same time
3D modeling and 3D printing of individual applicators was outsourced to a company registered for the manufacturing of medical devices with SLS technology. For the applicator production, a biocompatible polyamide PA2200 material was used, and the applicators were printed using a Formiga P100 3D printer. The applicator was delivered and sterilized 6 days after plan A. The treatment time was not prolonged due to the use of an individual applicator. There were no intra- and peri-operative complications.
Brachytherapy procedure and treatment planning
Before the first use of the 3D-printed ring applicator, a Varian bronchial catheter was inserted in the ring channel. Similarly, Varian guiding tube catheter was inserted in the tandem or vaginal cylinder central channel. Varian catheters were used to connect the applicators to the afterloader. In this way, it was ensured that the source capsule was always in contact only with Varian certified materials. As part of the quality control, the applicators were scanned on computer tomography (CT) in order to measure their internal structure. Additionally, active channel patency and geometry of the needle guiding holes were carefully checked.
At the time of BT, the exact needle insertion length was marked on the needle with a sterile marker before the applicator insertion. After the applicator insertion, parallel and oblique needles were inserted one by one through guiding holes to predefined depth. Their position was checked with transrectal and transabdominal ultrasound. The individual applicators were fixed in the same way as the standard applicators with vaginal tamponade and adhesive tape. The fixation of the needles in multichannel vaginal cylinder was performed with a handle at the end of the application (see explanation in Figure 2). Water-filled catheters were inserted into the ring and tandem applicators or in the central channel of the vaginal cylinder in order to visualize the applicator active channel on T2-weighted MR images. The same technique for the applicator reconstruction was used both in standard and individual applicators, in agreement with the GEC-ESTRO GYN recommendations on applicator reconstruction [29].
After applicator insertion, MRI scanning was performed. CTV-THR, GTV-T, and OARs were delineated, and the in situ applicator was reconstructed on MRI images. In the tandem and ring applicator, the starting point for dose optimization was standardized pear-shaped dose distribution with dose normalized to point A. The dwell times in the needles were normally limited to 20% of the dwell times of the standardized plan in order to prevent dose hot spots. In individual cases, the dwell time limit was increased to 30% of the standardized dwell times to compensate for suboptimal implant geometry. Dose optimization was performed in iterative steps by changing dwell positions and dwell times in the IC and IS parts of the implant. Reiteration was performed until the dose constraints for CTV-THR and OARs were met. In a multi-channel cylinder, the central channel dwell positions were loaded first, and the prescribed dose was normalized to the applicator surface in the central plane of the cylinder. Afterwards, dwell positions of the needles were loaded to cover the target volume with the prescribed dose. For the purpose of vaginal mucosa sparing, the needles were loaded at least 5 mm from the vaginal surface into the tissue. Three different types of individual implants are displayed in Figure 3.
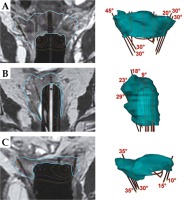
Fig. 3
MRI of three different types of 3D implants (left side) with corresponding CTV-THR (cyan color), with active channel source positions and angles for oblique needles (right side). A) Tandem, ring, and add-on for parallel and oblique needles; B) Multi-channel vaginal cylinder for the implantation of parallel and oblique needles; C) Ring for the interstitial implantation of parallel and oblique needles; B and C) Implants used in patients after hysterectomy
The cumulative dose aim (EBRT + 2 BT fractions) to the CTV-THR was D90 > 85 Gy EQD2 for cervical or recurrent endometrial cancer, and 82 Gy for vaginal cancer patient. For OARs, the D2cm3 EQD2 constraints were as follows: rectum, sigmoid, and bowel 70 Gy, and bladder 75 Gy. EQD2 was calculated using α/β = 10 for tumor and α/β = 3 for OARs. The estimated repair half-time for PDR was 1.5 hours. The aim per one BT fraction was CTV-THR V100 > 90% of the CTV-THR volume, and D98 > 80%, D90 > 100%, and D100 > 60% of the prescribed dose to D90 CTV-THR. Assuming an EBRT dose of 45 Gy with 1.8 Gy/fraction, the D2cm3 constraints for a single BT fraction were 15.1 Gy for the bladder and 13.4 Gy for the rectum, sigmoid, and bowel. If an EBRT dose of 50 Gy, 2 Gy/fraction was delivered, the D2cm3 constraints for a single BT fraction were 12.5 Gy for the bladder and 10.6 Gy for the rectum, sigmoid, and bowel.
For each patient, DVH parameters of three plans were compared: the virtual plan for designing individual applicator (plan A), the plan with the standard IC/IS applicator (plan B), and the plan with individual applicator (plan C). Plan A was created from the MRI from the first BT application, with standard applicator in situ. Virtual needles were introduced in the treatment planning system to obtain the best possible coverage of the CTV-THR, taking into account anatomical and geometrical limitations of the applicator. Plan A was never used for treatment. Plan C was created using the MRI from the second BT, with an individual applicator in situ. Plan B was created using the same MRI as plan C and a standard applicator. Standard applicator for plan B was defined as an individual applicator without oblique needles. Standard applicator for plan B for cervical cancer with uterus in situ was tandem and ring with parallel needles; for hysterectomized patient (recurrent endometrial, cervical, or vaginal cancer), the standard applicator was ring or vaginal cylinder with parallel needles. Plan B was not used for treatment either.
Statistics
The data were analyzed using IBM SPSS statistical software package version 23.0 (SPSS Inc., Chicago, IL, USA). P values of < 0.05 were considered statistically significant. The paired-sample t-test or Wilcoxon signed-rank non-parametric test for related samples was performed to test the difference.
The study was approved by the Republic of Slovenia National Medical Ethics Committee (approval number: 0120-282/2016-2) and by the Protocol Review Board at the Institute of Oncology Ljubljana (ERID-KESOPKR/63), and was in agreement with the Declaration of Helsinki. All the patients signed an informed consent form. The study was registered in the ClinicalTrials.gov database (NCT03466957).
Results
In our study, the mean number of needles that were used in the individual applicator was 9 ±2. The mean number of parallel needles was 5 ±2, and the mean number of oblique needles was 4 ±2 at 10 to 45-degree angle. The mean depth of needle implantation was 4 ±1.4 cm, for parallel needles 3 ±1.2 cm, and for oblique needles 4 ±1.4 cm. The maximal depth of needle implantation was 6.8 cm. The parallel needles were most often implanted in the 4-11 o’clock (clockwise) ring positions, while oblique needles were implanted in the 4-5 and 9-10 o’clock ring positions corresponding to the sites of the tumor infiltration.
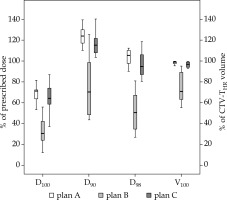
DVH parameters for all three plans of all patients per one BT fraction are presented in Table 2. All DVH parameters for CTV-THR were markedly improved with the use of individually designed 3D-printed applicators (Figure 4). If plan B was compared to plan C, all CTV-THR parameters (CTV-THR V100, D98, D90, and D100) and GTV-T D98 significantly improved (p < 0.001 for CTV-THR D90, V100, D98, and D100; p = 0.007 for GTV-T D98). They resulted in an increased mean CTV-THR DVH parameters: 22.3 ±13.4% for V100, 46.9 ±20.9% for D98, 44.6 ±26.7% for D90, 32.0 ±15.9% for D100, and 33.7 ±27.9% for GTV D98. CTV-THR V100, D98, D90, and D100 were ≥ 93.1%, ≥ 80.6%, ≥ 103.6%, and ≥ 37.4% in all patients, respectively. GTV-T D98 was more than 105% of the prescribed dose in all cases. Each CTV-THR DVH parameter in plan C had a smaller standard deviation compared to plan B.
Table 2
DVH parameters for CTV-THR (A) and OARs (B) for one BT fraction. The dose prescribed to D90 CTV-THR was 18.5 Gy for cervical or recurrent endometrial cancer, or 15 Gy for vaginal cancer patient (patient 9). Mean and standard deviation (SD) values are reported. The dose to OARs is presented as the percentage of the dose constraints. Assuming an EBRT dose of 45 (50) Gy with 1.8 (2) Gy/fraction, the D2cm3 constraints for a single BT fraction are 15.1 (12.5) Gy for bladder and 13.4 (10.6) Gy for rectum, sigmoid, and bowel. If the OAR (bowel) was not visible on MRI images, the cell is empty
A
Table 2
B
Fig. 4
DVH parameters for CTV-THR D90, D98, and D100 in percentage of prescribed dose to D90 CTV-THR and V100 in percentage of CTV-THR volume. The dose prescribed to D90 CTV-THR was 18.5 Gy or 15 Gy per fraction in vaginal cancer patient (patient 9, Table 2). A) Plan A: virtual plan for designing individual applicator; B) Plan B with standard applicator; C) Plan C with 3D-printed individual applicator
As expected, the difference in DVH parameters between plan A and plan C was non-significant (p > 0.05). In most cases, the DVH parameters for CTV-THR in plan A were the best that could be achieved for CTV-THR coverage in a given patient, partly because no special consideration was given to OARs. The primary aim of plan A was the improvement of spatial distribution of parallel and oblique needles in order to obtain the best possible CTV-THR coverage and not the dose to OARs. The difference between DVH parameters in favor of plan A for CTV-THR D98, D90, V100, D100, and GTV-T D98 were 3.4 ±16.2%, 5.6 ±12.7%, 1.3 ±3.5%, 0.2 ±9.0%, and 27.3 ±33.9%, respectively. The dose constraints for OARs were exceeded in most cases (6/9 patients) in plan A, while respected in all patients in plan B and in most patients in plan C. The mean increase in the dose constraints for D2cm3 per one BT fraction for the bladder, rectum, sigmoid and bowel when comparing plans C and B was 4.3 ±5.5%, 6.6 ±4.9%, 10.7 ±9.1%, and 4.4 ±9.2%, respectively (p < 0.05 for the bladder, rectum, and sigmoid; p = 0.2 for the bowel). The mean increase in the dose constraints for D2cm3 per one BT fraction between plan A and C for the bladder, rectum, sigmoid and bowel was 15.2 ±12.2%, 26.1 ±38.9%, 10.6 ±30.9%, and 34.2 ±27.9%, respectively (p < 0.05 for bladder and bowel; p > 0.05 for rectum and sigmoid). In two patients, the dose to the bladder and rectum slightly increased with the use of individual applicators (plan C), without surpassing the cumulative EBRT + 2 BT EQD2 dose.
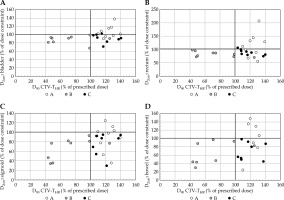
The mean volume of CTV-THR was 49.9 ±31.1 cm3. The lowest CTV-THR volume was only 5.2 cm3 in a patient with recurrent cervical cancer after hysterectomy, and the highest was 96.7 cm3 in a patient with primary cervical cancer. The benefit of individual applicators was seen in both small and large tumor volume, and was strongly related to the topography of the tumor. In Figure 5, the relation between percentage of the dose constraints to OARs and percentage of the prescribed dose to CTV-THR D90 is visualized for all OARs. Plan C offered optimal results for CTV-THR coverage in relation to the dose to OARs. CTV-THR was well covered in plan A, but the dose to OARs was exceeded in 6/9 of these patients for bladder, in 5/9 for rectum, and in 4/9 for sigmoid and bowel. OARs dose constraints were met in plan B, except for one patient, while CTV-THR was underdosed for all patients, except one.
Fig. 5
A-D) Relation between percentage of the dose constraints to OARs and percentage of prescribed dose to D90 CTV-THR for plan A, B, and C for D2cm3 A) bladder; B) rectum; C) sigmoid and D) bowel. Assuming an EBRT dose of 45 (50) Gy with 1.8 (2) Gy/fraction, the D2cm3 constraints for a single BT fraction are 15.1 (12.5) Gy for bladder and 13.4 (10.6) Gy for rectum, sigmoid, and bowel. DVH constraints are indicated with horizontal and vertical black line
Discussion
IGABT has become a standard of care in patients with advanced cervical cancer. It has significantly improved local and pelvic control rates for all cervical cancer stages, with a survival benefit of at least 10%. Major morbidity was decreased by about 50% [1,4,5]. The 3D printing technology is a promising new approach in IGABT and offers the possibility of improving standard BT applicators available on the market as well as developing new ones. A potential benefit of 3D printing technology is to escalate the dose to the target and de-escalate the dose to OARs. 3D-printed applicators are useful tools for guiding needle implantation at different angles and directions. Freehand needles can be used as well, but it is difficult to implant them accurately at predefined angels and to a certain location. However, only a limited number of reports on the use of 3D technology in BT have been published up to date [14,15,17,20,21,30,31]. For intact cervical cancer, CTV-THR D90 dose of ≥ 85 Gy in 7 weeks leads to 3-year local control rates of > 86% in large size CTV-THR and > 93% in intermediate size CTV-THR. In advanced tumors, appropriate target coverage can only be achieved with combined IC/IS application [1,4,6,7,9,13,32]. RetroEmbrace study outcomes showed that the dose to CTV-THR in cervical cancer decreased significantly with advanced stage from the mean D90 of 93 Gy in stage IB to 78 Gy in stage IVA [1]. It was also shown that half of the patients with volumes CTV-THR of more than 30 cm3 received doses of less than 85 Gy [33]. Suboptimal local control is predicted if the dose to CTV-THR is less than 85 Gy. A further study demonstrated that a dose escalation from 75 Gy to 85 Gy resulted in increased local control of 3% in limited/intermediate size volume, and of 7% in large size volume CTV-THR [13]. It was proved that combined IC/IS technique improves the therapeutic ratio in locally advanced cervical cancer. IS technique is an optimal solution for boosting involved parametrium [12].
In our study, the principles for cervical cancer IC/IS approach were used also for other gynecological cancers. With individual applicators, the mean D90 to CTV-THR in all patients increased by 44.6 ±26.7% compared to standard applicator. According to the local tumor control probability (TCP) model, about 15% better local control at 3 years due to the increment in the dose can be expected in patients with locally advanced cervical cancer [13]. On the other hand, the dose to OARs in most of the cases was below institutional dose constraints, which were lower than those used in most other IGABT studies, except for the rectum within the dose limits proposed in the EMBRACE II study [3,4,32,33,34]. It was shown that the rectal dose of ≥ 75 Gy is associated with a 12.5% risk of fistula at 3 years, and the dose of ≤ 70 Gy limited G2-G4 proctitis to ≤ 10.2% [35]. Findings from the Embrace I study suggest limiting the bladder D2cm3 to ≤ 80 Gy [33]. With dose constraints for the bladder in our study, the probability of G2-G4 bladder late morbidities was estimated to be up to about 20% [36]. With the sigmoid and bowel dose limit for D2cm3 < 70 Gy, no additional side effects are to be expected apart from those mentioned in other studies [4,33,34]. The mean percentage of the dose constraints for D2cm3 per one BT fraction was 92.1 ±10.1 for the bladder and 89.3 ±10.5 for the rectum. If the two BT fractions with the same dose were to be delivered to both fractions, the dose to OARs would still be below the dose constraints proposed for OARs.
However, there are some limitations of the method and study. An individualized 3D-printed approach is needed for a minority, in our experience about 5%, of patients with gynecological cancers (primary or recurrent cervical, endometrial or vaginal cancer), who are candidates for BT. For the majority of patients, the standard IC/IS applicators offer optimal results for the target coverage.
Based on the results from this study, the benefit of using individual applicators is to be expected for all stages and for all tumor volumes. It is not reliant only on the tumor volume or the tumor stage, but also on the anatomy of the tumor and nearby structures (proximity of OARs, blood vessels, narrow vagina, etc.). Patients with both small and large tumors would benefit from individual applicators due to difficult topography. The important step in designing the individual applicator is choosing the right type of standard applicator that suits the patient’s anatomy and tumor’s topography. Three types of standard applicators were used in our study: in patients with uterus in situ, a tandem with a ring or a multichannel vaginal cylinder with a tandem, and in hysterectomized patients, a ring or a cylinder depending on the tumor extension to vagina. The next important step is reproducing the applicator position for all insertions. If the patient has a uterus, the uterine channel facilitates reproducible applicator positioning in all applicator insertions. Consequently, it is more difficult to position the applicator in hysterectomized patients. Ultrasound can be useful for the purpose of accurate applicator positioning.
Future improvement of the technique would be the development of a computer program that would automate the design of individualized applicator. With the help of a dedicated software, the standard type of applicator inserted into the patient would be chosen from the library of applicators. Based on the target and OARs delineated on the MRI, virtual needles would be added to the standard applicator, resulting in an individual applicator. Such an automated process would speed up the design of individualized applicator. It would also enable optimal applicator geometry for each individual patient.
Patients anatomically unsuitable for the use of individual BT applicators are candidates for the perineal template BT, other techniques (EBRT boost, intraoperative BT, stereotactic, or proton therapy) may be considered as well [37,38,39,40]. The process of manufacturing individual applicators is not automated and demands skillful and dedicated staff. Moreover, not all materials on the market designated for the use in medicine have proper characteristics for an applicator’s production. The weakness of our study is also a low number of the patients, which is mainly due to the eligibility criteria.
Conclusions
This study focused on designing and testing 3D-printed applicators for clinical practice. We confirmed that an improvement of CTV-THR DVH parameters in advanced gynecological tumors, where the need for IC/IS BT is mandatory, could be achieved with the help of individualized 3D-printed applicators without exceeding the dose to OARs. A higher D90 can result in better tumor control probability. However, long-term results with regard to local control and survival should be evaluated in further studies, preferably with a larger number of patients.



