Summary
This study evaluated the relationship between the dicrotic notch index (DNI) and hemodynamic, echocardiographic, and clinical parameters in patients with pulmonary hypertension (PH). A retrospective analysis of 76 patients diagnosed with pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, or normal pulmonary artery pressure who underwent right heart catheterization was conducted. DNI showed significant correlations with key hemodynamic parameters and was identified as an important predictor of PH. The study suggests that DNI could be a marker in assessing PH and useful in evaluating pulmonary arterial stiffness and resistance.
Introduction
Pulmonary hypertension (PH) is predominantly characterized by the loss of arterial bed elasticity, leading to progressive remodeling and vascular obstruction that result in elevated pulmonary artery pressure (PAP) and increased pulmonary vascular resistance (PVR) [1]. In PH, the right ventricular (RV) load is influenced by factors such as arterial elasticity and wave reflection, in addition to vascular resistance [2]. While vascular resistance can be assessed through right heart catheterization (RHC), pulmonary arterial elasticity and wave reflections cannot be evaluated using conventional RHC [3]. Therefore, the dicrotic notch index (DNI), which captures the characteristics of the reflected wave and vascular elasticity, becomes important [4]. The elastic properties of arteriolar walls generate reflected waves, which are directly related to vascular stiffness and compliance [3, 4]. Increased resistance in the distal pulmonary arteries induces significant changes in the pulmonary artery pressure waveform [5]. In PH, the reduction in arterial elasticity is a key feature. The DNI, which captures the characteristics of the reflected wave, has been identified in previous studies as an indicator of arterial elasticity [4, 6]. Considering this, we hypothesize that DNI correlates with pulmonary artery pressures and resistance in PH due to its reflection of vascular stiffness and compliance.
Aim
The aim of this study was to evaluate the relationship of DNI with PVR, PAPs and other RHC parameters, as well as echocardiographic and clinical parameters in patients with PH.
Material and methods
Patient selection
This retrospective study included patients with a prediagnosis of PH who underwent RHC at two specialized PH centers, with data collected from electronic health records and patient charts between February 2019 and February 2023. Patients aged 18 and older with normal RHC values and diagnosed with PH clinical class I (PAH) or class IV (CTEPH) were included. Exclusions were made for those with incomplete records, PH groups II, III, and V, those receiving PAH-specific therapy, post-pulmonary endarterectomy CTEPH patients, those with atrial flutter/fibrillation, moderate to severe pulmonary stenosis, and severe pulmonary insufficiency. These exclusions were necessary due to distinct hemodynamic profiles influenced by conditions such as left heart dysfunction, chronic lung diseases, anemia, and hypoxia. Such conditions could obscure true pulmonary vascular resistance and compliance calculations. Diagnoses of group I and group IV PH were confirmed according to current clinical guidelines, involving imaging studies, hemodynamic criteria, and other relevant clinical assessments.
Right heart catheterization procedure and echocardiographic measurements
RHC was performed on 76 patients with a pre-diagnosis of PH, utilizing the right internal jugular or femoral vein under ultrasound guidance with a 7F sheath and a triple lumen Swan-Ganz catheter. PAP waveform recordings were obtained using a Siemens Sensis Hemodynamic System. Cardiac output (CO), cardiac index (CI), and PVR were calculated via the Fick method, using an estimated oxygen consumption (VO2) value of 3.5 ml O2 per kg per minute. Hemodynamic parameters including sPAP, mPAP, dPAP, and pulmonary capillary wedge pressure (PCWP) were measured. Patients with mPAP above 20 mm Hg were categorized under the pulmonary hypertension group, while those with mPAP at or below 20 mm Hg were considered to have normal pulmonary artery pressure. Echocardiographic evaluations were conducted by experienced operators following standard protocols to ensure high-quality imaging and accurate right heart assessment.
Pulmonary artery waveform measurements of dicrotic notch index
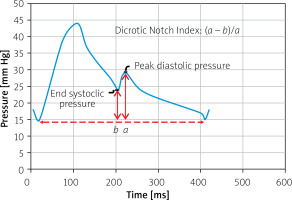
Pulmonary artery pressure waveforms were analyzed using WebPlotDigitizer software. DNI was defined as the difference between the peak diastolic pressure and end systolic pressure and divided by the difference between the peak diastolic pressure and end diastolic pressure (Figure 1).
Statistical analysis
Categorical variables were expressed as percentages and analyzed with the χ2 test, while the Kolmogorov-Smirnov test assessed the normality of continuous variables. Pearson correlation coefficient (r) values and p-values were used to analyze correlations between DNI and PVR, PAPs, and other RHC, echocardiographic, and clinical parameters. For comparisons of echocardiographic, hemodynamic, and DNI variables between the normal, PAH, and CTEPH groups, ANOVA or Kruskal-Wallis tests were used depending on normality, with post-hoc tests performed to identify specific group differences. Logistic regression analyses identified predictors of DNI, with odds ratios (OR) and 95% confidence intervals (CI) calculated. ROC analysis determined the sensitivity and specificity of DNI in predicting PH. Statistical significance was set at p ≤ 0.05, and all analyses were performed using SPSS software version 24 (IBM Corp., NYC, USA).
Results
The study analyzed 76 patients, divided into two main groups: 14 with normal pulmonary artery pressure and 62 with confirmed PH. The mean age was 54.3 ±11.2 years in the normal group and 52.8 ±15.4 years in the PH group, with the number of male patients being 6 (42.8%) and 27 (43.5%) in each group, respectively. Detailed baseline clinical characteristics, including comorbidities, medication use, and laboratory parameters, are summarized in Table I. Table II further categorizes the PH patients into two subgroups: PAH and CTEPH, presenting detailed echocardiographic, RHC measurements, and DNI. These results show differences in hemodynamic profiles between the normal, PAH, and CTEPH groups, with p-values provided for all relevant parameters. Notably, DNI demonstrated a statistically significant difference between the groups (p = 0.002).
Table I
Baseline clinical characteristics of study patients
[i] NA – not applicable/not measured, DPN CCB – dihydropyridine-derived calcium channel blockers, ASA – acetylsalicylic acid, OAC – oral anticoagulant, MRA – mineralocorticoid receptor antagonist, WHO-FC – World Health Organization functional class, WBC – white blood cells, 6MWT – 6-minute walk test, pro-BNP – pro-brain natriuretic peptide.
Table II
Echocardiographic and catheterization measurements, and dicrotic notch index values
[i] EF – ejection fraction, TR vel – tricuspid regurgitation velocity, TAPSE – tricuspid annular plane systolic excursion, PaccT – pulmonary acceleration time, PCWP – pulmonary capillary wedge pressure, RV – right ventricle, RA – right atrium, sSO2 – systemic oxygen saturation, pSO2 – pulmonary artery oxygen saturation, mvSO2 – mixed venous oxygen saturation, CO – cardiac output, CI – cardiac index, PVR – pulmonary vascular resistance.
The correlation analysis demonstrated that the DNI had significant positive correlations with several RHC parameters: sPAP (r = 0.972, p < 0.001), dPAP (r = 0.876, p < 0.001), mPAP (r = 0.987, p < 0.001), right atrial mean pressure (RA) (r = 0.741, p = 0.018), and PVR (r = 0.814, p < 0.001). There were significant negative correlations with cardiac index (CI) (r = –0.573, p = 0.012) and pulmonary artery oxygen saturation (pSO2) (r = –0.516, p = 0.043). No significant correlations were found with (PCWP), cardiac output (CO), or 6-minute walk test (6MWT) distance. Among echocardiographic parameters, DNI showed significant positive correlations with tricuspid regurgitation velocity (TR vel) (r = 0.770, p = 0.018) and sPAP (r = 0.701, p = 0.006). These correlations are detailed in Table III.
Table III
Correlation of right heart parameters with dicrotic notch index
[i] RHC – right heart catheterization, sPAP – systolic pulmonary artery pressure, dPAP – diastolic pulmonary artery pressure, mPAP – mean pulmonary artery pressure, RA – right atrium mean pressure, PCWP – pulmonary capillary wedge pressure, PVR – pulmonary vascular resistance, CO – cardiac output, CI – cardiac index, TR vel – tricuspid regurgitation velocity, PaccT – pulmonary artery acceleration time, 6MWT – 6-minute walk test.
In the univariable logistic regression analysis, DNI was a significant predictor of PH with an odds ratio (OR) of 1.100 (95% CI: 1.048–1.155, p < 0.001). Similarly, echo sPAP (OR = 1.066, 95% CI: 1.025–1.108, p < 0.001) and TR vel (OR = 1.187, 95% CI: 1.053–1.338, p = 0.005) were significant predictors. In the multivariable logistic regression analysis, DNI remained a significant predictor (OR = 1.061, 95% CI: 1.007–1.118, p = 0.025), while echo sPAP (OR = 0.996, 95% CI: 0.985–1.007, p = 0.48), NT-proBNP (OR = 1.000, 95% CI: 0.999–1.000, p = 0.46), and TR vel (OR = 1.192, 95% CI: 0.895–1.588, p = 0.22) were not significant in the multivariable model. These results are presented in Table IV.
Table IV
Univariable and multivariable analysis of predictors for pulmonary hypertension
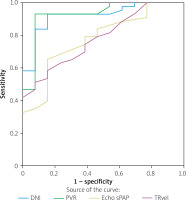
The ROC curve analysis for DNI showed an area under the curve (AUC) of 0.922, indicating excellent discriminatory power for predicting pulmonary hypertension. This was compared with PVR, echo sPAP, and TR vel, with DNI demonstrating superior performance (Figure 2).
Discussion
In this study, we evaluated the relationships between the DNI and various hemodynamic, echocardiographic, and clinical parameters in patients with PH. Our results demonstrate that DNI is significantly correlated with key hemodynamic parameters – PAPs (sPAP, mPAP, and dPAP) and PVR – highlighting its potential as a marker of pulmonary arterial stiffness and resistance.
PH is characterized by increased pressure and/or volume load on the pulmonary vasculature, leading to fibrosis and loss of elasticity in the vessel walls [7, 8]. Additionally, there is an increase in vascular resistance due to distal arterial vascular obstruction, which further elevates pulmonary artery pressures and exacerbates the paradoxical pressure load [9]. This condition creates a combined load on the RV involving pulmonary vascular resistance, wave reflections, and arterial elasticity [10]. The assessment of RV function, particularly through invasive evaluation with RHC, is critical for clinical management [11]. However, conventional RHC assesses PVR but does not measure pulmonary arterial elasticity or wave reflections [2]. Previous studies have shown that the dicrotic notch and DNI are associated with arterial elasticity and arterial stiffness [4, 6]. Thus, evaluating DNI, which captures elasticity and the reflected wave’s theoretical model via the dicrotic notch of the pulmonary artery, is crucial in our study. In this study, the significant positive correlations found between DNI and RHC parameters, such as sPAP, dPAP, mPAP, RA, and PVR, suggest that higher DNI values are associated with increased arterial stiffness and resistance. These findings align with the understanding that PH is characterized by increased pulmonary artery pressure and resistance, often due to the loss of arterial elasticity. The strong correlation with mPAP and PVR underscores the utility of the DNI in reflecting the hemodynamic burden imposed on the RV in PH patients. Additionally, the DNI could complement conventional measures such as PVR and PAP by offering insights into the state of the distal pulmonary vasculature, particularly the arterial stiffness and wave reflections impacting RV afterload.
In the PAH and CTEPH groups, more patients were treated with calcium channel blockers (CCBs) and β-blockers. While these medications are known to reduce arterial stiffness in the systemic circulation [12], their impact on pulmonary arterial stiffness remains unclear. Despite such treatments, patients in our study still showed elevated PAP and PVR, with DNI increasing in correlation with these values. This suggests that CCBs and β-blockers may influence pulmonary arterial stiffness, but their effects might vary depending on changes in PAP and PVR, with DNI potentially adjusting in parallel.
Additionally, both the pulmonary arteries and the aorta act as elastic reservoirs during systole, storing some of the ejected blood, which is then forced out into the peripheral vessels during diastole (Windkessel effect) [13]. This gradual release of blood contributes to the formation of the dicrotic notch as the aortic and pulmonary valves close and a brief backflow occurs [14]. In PH, the pulmonary arteries lose their elastic properties, leading to decreased vascular compliance and a reduced amplitude of the dicrotic notch in the pulmonary artery pressure waveform [15]. This phenomenon was confirmed in our study, where the DNI showed a correlation with both PAPs and PVR, indicating increased arterial stiffness and resistance.
In our study, the DNI was found to be higher in the PAH group compared to the CTEPH group, consistent with other key RHC measurements. This finding aligns with the expectation of increased arterial stiffness, particularly in PAH subgroups where inflammatory processes result in fibrosis. For instance, in PAH subgroups associated with connective tissue diseases such as scleroderma and rheumatoid arthritis, arterial inflammation and fibrosis are likely to significantly alter arterial waveforms due to increased stiffness. As demonstrated by Liu et al. [16], in these subgroups characterized by prominent inflammation, arterial stiffness increases, which subsequently affects wave reflection and pulmonary hemodynamics. Therefore, it can be anticipated that significant changes in arterial waveforms may develop in these PAH subgroups.
Univariable logistic regression analysis identified the DNI as a significant predictor of PH, suggesting its utility in distinguishing between PH and non-PH patients. The multivariable analysis further supports the significance of DNI, indicating that even when accounting for other factors, the DNI remains a robust predictor of PH. ROC curve analysis further demonstrated the DNI’s excellent discriminatory power for predicting PH, surpassing other parameters such as PVR, echo sPAP, and TR velocity.
Our findings suggest that the DNI could serve as an invasive parameter to assess pulmonary arterial stiffness and wave reflections, enhancing the diagnostic and prognostic capabilities in PH by providing a more comprehensive understanding of its impact on pulmonary vasculature and right heart function. Moreover, significant correlations with echocardiographic parameters such as TR velocity and sPAP, as well as with NT-proBNP and 6MWT distance, further validate the DNI’s clinical utility, linking it to both hemodynamic and clinical status in PH patients. Considering recent advancements in balloon pulmonary angioplasty (BPA) for CTEPH [17], refined hemodynamic indices such as the DNI could be valuable for assessing changes in pulmonary arterial stiffness before and after BPA. The effectiveness of DNI may be validated by examining its correlation with hemodynamic, echocardiographic, and clinical parameters in pre- and post-BPA evaluations.
Study limitations. The retrospective nature of the study may result in biases related to data collection and patient selection. The findings may not be widely generalizable due to the relatively small sample size from only two centers. To ensure accurate comparisons of PVR and other RHC results, patients with comorbidities that could affect RHC measurements were excluded, potentially limiting the scope of the study. Additionally, the use of CCBs and β-blockers among patients in the PH group could have influenced the DNI measurements, as these medications are known to affect arterial stiffness, although their specific impact on pulmonary circulation remains unclear. Further research with larger, multicentric cohorts is needed to validate these findings and to establish standardized protocols for DNI measurement.
Conclusions
This study demonstrates that the DNI shows a strong correlation with key hemodynamic parameters such as PVR and mPAP, underscoring its potential as an important parameter in the assessment of PAH and CTEPH patients. Additionally, DNI exhibited statistically significant correlations with other RHC parameters, echocardiographic measurements, and clinical indicators, including sPAP, RA, and TR velocity. These findings highlight the utility of DNI as a comprehensive tool for evaluating pulmonary arterial stiffness and resistance, offering enhanced diagnostic insights beyond conventional RHC metrics. Further research is needed to validate these results in larger, multicentric cohorts and to establish standardized protocols for DNI measurement in clinical practice.