Purpose
Prostate cancer (PCa) is the most common cancer diagnosed in Canadian men, with an estimated 24,600 new cases and 4,600 deaths in 2022 [1]. The high diagnosis-to-mortality ratio emphasizes the important heterogeneity of PCa, which poses challenges in accurately predicting patient outcomes and individualizing treatment [2]. This underscores the importance of accurately stratifying patients to optimize treatment of those with an aggressive disease, and reduce over-treatment of indolent cancers. To aid clinicians in making informed treatment decisions, various risk stratification tools have been developed. However, many of these tools have been designed to predict surrogate end points, such as biochemical recurrence, with no clear evidence of causal relation to direct clinical benefit and patient outcomes; neither have they been shown to be predictive of benefit to a specific treatment [3, 4]. Moreover, most of these tools were developed in a selected and radically treated population rather than as a prognostic tool for untreated men [5].
The D’Amico classification system and its derivatives, including the older 3-tier NCCN stratification system, are some of the most used stratification tools for PCa. These tools use prostate specific antigen (PSA) at time of diagnosis, clinical tumor stage, and Gleason score information to classify individuals into low-, intermediate-, and high-risk groups. Contemporarily, these tools lack clinical and pathological granularity, leading to a sub-standard discrimination of risk populations and to an important heterogeneity in clinical outcomes [5-7]. In recent years, the cancer of the prostate risk assessment (CAPRA) score and the newer 5-tier NCCN classification system have emerged as more finely tuned prognostic tools, which can allocate patients into more accurate risk categories.
The CAPRA score, incorporating percentage of positive biopsies and patient age at time of diagnosis, was initially designed for pre-operative setting [8]. The score has been externally validated in a neoadjuvant population, and has been linked to PCa clinical outcomes, including bone metastasis and cancer-specific mortality [9, 10]. It has also been shown to have better prognostic performance in predicting prostate cancer death than D’Amico-derived systems [5]. The newer 5-tier NCCN classification system further refines risk stratification by considering invasion patterns and number of positive biopsies [7]. This tool has shown promising results in predicting PCa relevant outcomes, including disease recurrence and metastasis in salvage setting [11].
While both these classification tools identify high-risk prostate cancer populations with notable overlap, discrepancies in their prognostic outcomes at 5 and 10 years have been observed [5]. Currently, androgen receptor axis-targeted therapy (ARAT) is used for high-risk prostate cancer, and is based primarily on NCCN classification. This underscores the need to evaluate other tools, such as CAPRA, to improve management strategies for high-risk PCa patients and potentially broaden ARAT indications. This study aimed to assess the prognostic significance of the CAPRA score and the 5-tier NCCN classification system in PCa patients as well as their potential to assist in informed treatment decisions.
Material and methods
Study population
Four hundred seventy-one patients with high-risk PCa according to the 3-tier NCCN risk classification were retrospectively enrolled in this study. They received external beam radiation therapy (EBRT) and high-dose-rate brachytherapy (HDR-BT) boost at our institution between 1999 and 2018. Patients were included if they received concurrent and adjuvant androgen deprivation therapy (ADT) in accordance with clinical guidelines [4]. ADT was administered using either GnRH analogs injections overlapping with short-term bicalutamide use, or GnRH antagonists. The study outcomes included biochemical relapse (BCR), metastasis incidence, and overall survival (OS).
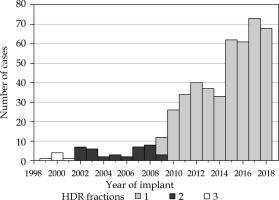
Partly, due to the long history of using EBRT + HDR boost for over 20 years at our institution, there have been slight variations in techniques, total doses, and doses per fraction to the prostate. Most of our cohort (n = 395, 84% of patients) received EBRT of 44 Gy in 22 fractions or 45 Gy in 25 fractions, with a subsequent 15 Gy HDR implant boost in a single fraction. Variations included a minority of individuals receiving 36 to 37.5 Gy in 12 to 15 fractions (n = 14, 3% of patients), or an alternate HDR boost of 19 to 20 Gy in two fractions (n = 38, 8% of patients) (Table 1).
Table 1
Patient and disease characteristics
Radiation and treatment characteristics
The institutional practice for EBRT was to irradiate the prostate and pelvic nodes using either 3-dimensional conformal (3D-CRT) or intensity-modulated radiation therapy (IMRT) technique. Implanted fiducial gold markers were used for daily image-guided radiation therapy (IGRT). HDR-BT boost was given within two weeks of EBRT. Computed tomography (CT) or ultrasound scans (US) were obtained post-operatively for planning, using inverse-planning simulating annealing algorithm (IPSA).
Statistical analysis
Descriptive statistics were presented as means ± standard deviation (SD), or median with interquartile range (IQR). Competing risk survival analysis were used to compare patients with a CAPRA score < 6 and ≥ 6 for BCR and metastasis incidence, with death as a competing event. The same analyses were performed for NCCN high- and very high-risk patients. Significative difference between groups were assessed by Gray’s test. Kaplan-Meier analysis with log-rank tests were performed to assess and compare OS between groups. Prognostic factors associated with BCR and metastasis incidence were evaluated using Fine and Gray’s models, while for OS, Cox regression modeling was applied. Subsequently, ROC curves were utilized to compare the performance of CAPRA model with the NCCN stratification tool for BCR, metastasis incidence, and OS. Sensitivity analysis was not performed per se, but all statistical models were built by conditional methods to ensure that only relevant variables were included. Statistical analyses were performed using IBM SPSS v. 28.0 and R 4.1.3, with the cmprsk [12] and prodlim packages.
Results
The current study included 471 patients with the 3-tier NCCN high-risk PCa classification who underwent EBRT and HDR-BT boost with ADT at our institution between 1999 and 2018 (Figure 1). Patients’ and disease characteristics are detailed in Table 1. The median age at implant was 71 years (IQR: 66-76 years), with a median follow-up time of 72 months (IQR: 46-90 months). Most patients had stage T1-T2A disease (53.3%), a pre-treatment PSA value of less than 10 ng/ml (46.8%), and a Gleason score of 8 (58.1%). Four hundred fifty-three patients could be evaluated according to the newer 5-tier NCCN risk classification and among them, 183 (40.4%) were classified as having very high-risk disease [4].
Fig. 1
Number of high-dose-rate (HDR) brachytherapy cases performed each year at our institution between 1999 and 2018 according to number of fractions
Of the 471 patients, 446 had complete data for the calculation of their CAPRA score. Patients with missing data on staging and dose regimes were removed from the analysis. The scores ranged from 3 to 10, with the highest frequencies at 5 (125 patients, 25.7%) and 6 (113 patients, 23.2%). Although all patients were considered high- or very high-risk according to the NCCN classification, 268 (60.1%) had a CAPRA score ≥ 6, which is the high-risk indicator in this system. As discussed above, different techniques and prescription doses were utilized throughout time, with most common fractionation used for EBRT of 44 Gy in 22 fractions, with a subsequent HDR-BT boost of 15 Gy in a single fraction. To facilitate the comparison of radiation doses given at different dose per fraction for EBRT and brachytherapy regimes, we converted the radiation doses of EBRT and HDR boost to the equivalent dose in 2 Gy fractions (EQD2) [13], using an α/β ratio of 1.93 Gy for prostate cancer [14]. The median prescribed duration of ADT was 24 months for the entire cohort (IQR: 12-36 months).
Competing risks analyses were performed to assess clinical outcomes, with death acting as the competing event for BCR and metastatic progression. The cumulative incidence of BCR at 5 and 10 years post-treatment was 6.0% and 10.2%, respectively. During the same time intervals, 4.3% and 5.5% of patients had progressed to a metastatic disease. Overall survival was analyzed using the Kaplan-Meier method, and the 5- and 10-year incidence of death were 10.2% and 35.5%, respectively.
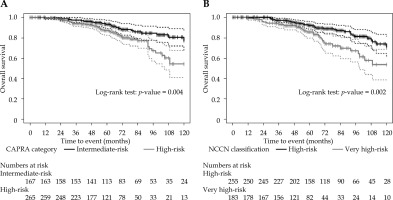
When patients were re-stratified using the CAPRA score, the cumulative incidence of BCR was significantly higher for those with a score ≥ 6 (Figure 2A). The 10-year BCR cumulative incidences were 5.3 ±2.4% and 14.2 ±3.3% for patients with a CAPRA score < 6 vs. ≥ 6 (Gray’s test, p = 0.0099), respectively. The same tendency was observed with the NCCN very high-risk classification (Figure 2B). The 10-year BCR cumulative incidences were 6.8 ±2.1% and 18.1 ±5.2% for patients classified as high-risk vs. very high-risk, respectively (Gray’s test, p = 0.0096).
Fig. 2
Cumulative incidence plots for biochemical relapse (BCR) according to CAPRA score (A) or NCCN classification (B). Metastasis cumulative incidence depicted by CAPRA score (C) and NCCN classification (D)
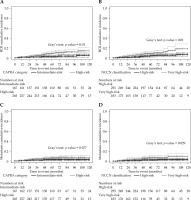
The 10-year metastasis incidence rates were 2.5 ±1.2% and 7.2 ±1.9% for patients with a CAPRA score < 6 or ≥ 6, respectively (Figure 2C). However, the difference did not reach statistical significance (Gray’s test, p = 0.077). On the other hand, high- and very high-risk patients grouped according to the 5-tier NCCN classification had significantly different 10-year metastasis incidence rates of 3.3 ±1.3% and 9.8 ±3.3%, respectively (Gray’s test, p = 0.029) (Figure 2D).
In terms of OS, both CAPRA and the 5-tier NCCN classification had significant differences for their higher-risk groups (Figure 3). For CAPRA, 10-year OS was 77.1 ±5.3% and 54.2 ±6.8% for patients with a score < 6 vs. ≥ 6, respectively (log-rank test, p = 0.004). Similarly, for the 5-tier NCCN, 10-year OS was 71.2 ±5.3% and 53.6 ±7.7% for patients classified as high-risk and very high-risk, respectively (log-rank test, p = 0.002).
When evaluating individual clinical characteristics, pre-treatment PSA was found to be the only clinical characteristic significantly associated with BCR (sHR = 1.01, p = 0.028). Furthermore, a CAPRA score ≥ 6 and belonging to the very high-risk group in the 5-tier NCCN classification were both significantly associated with BCR, with sHRs of 3.04 (p = 0.015) and 2.53 (p = 0.013), respectively (Table 2).
Table 2
Fine and Gray sub-distribution hazard models for biochemical relapse
In terms of metastasis incidence, no individual clinical characteristics were significantly associated with increased risk in the same cohort (Table 3). As for the risk stratification methods, both CAPRA and the fivetier NCCN had similar sHRs of 2.60 and 2.71, respectively, but only the NCCN reached statistical significance (p = 0.037 vs. p = 0.094 for CAPRA).
Table 3
Fine and Gray sub-distribution hazard models for metastasis incidence
The associations of clinical characteristics with OS were assessed using Cox proportional hazard regression models (Table 4). Univariate analysis identified only increasing age to be correlated with a decreased OS. To control for age, models, such as Gleason grade groups or tumor stage were made, with age as a covariate. Tumor stage T3 was found to be significantly associated with OS (HR = 1.88, p = 0.045). Both, having a CAPRA score ≥ 6 and belonging to the very high-risk group in the 5-tier NCCN stratification system, had similar HRs of 2.11 (p = 0.005) and 2.10 (p = 0.002) for mortality. Additionally, in these individuals, both biochemical relapse and progression to metastatic disease increased the risk of death, with HRs of 3.51 and 4.24 (p < 0.001), respectively.
Table 4
Cox proportional hazard models for overall survival
Finally, the ability of the CAPRA score and the 5-tier NCCN system were compared using ROC curve analyses, to predict for biochemical relapse, metastasis incidence, and death at 10 years post-treatment. While the maximal area under the curve (AUC) attained was 0.591 for metastasis incidence with CAPRA ≥ 6, no AUC reached statistical significance, and they were very similar between both the systems.
Discussion
Many of the stratification tools for PCa commonly used today have been developed to predict surrogate end points, with no strong evidence of causal relation to direct clinical benefit and patient outcomes. Moreover, they have been developed in a selected and radically treated population, rather than as a prognostic tool for untreated men [3, 5].
Research has demonstrated that a higher CAPRA score (above six) serves as a robust predictor of recurrence and mortality in post-operative setting in this population [10]. The CAPRA score has also been found to have superior prognostic performance in predicting prostate cancer death than D’Amico-derived systems [5]. Meanwhile, the 5-tier NCCN classification system has shown promising results in predicting relevant outcomes of PCa, including disease recurrence and metastasis in salvage setting [11]. This prompts the question of whether these tools can be utilized in a clinical setting to more effectively guide the diagnosis and management of higher-risk PCa patients. To the best of our knowledge, no study has directly compared the 5-tier NCCN classification system and the CAPRA score as prognostic tools in high-risk PCa patients treated with EBRT, HDR-BT, and ADT in a mature cohort. While significant overlap exists for the higher risk patients classified by either tool, some discrepancies in prognostic values have been observed in another study [5].
The results presented here show that there exists a benefit from risk re-stratification in PCa patients who were previously classified as having a high-risk disease according to the 3-tier NCCN classification system. Indeed, no single clinical characteristic could clearly indicate which patients were more at risk of treatment failure, but those classified in the higher tier of both composite indices proved to have significantly increased sHR or HR for biochemical-relapse, metastasis incidence (NCCN only had p-value < 0.05), and OS.
Our analyses showed that both the stratifying methods produced nearly identical results in most assays. Although the CAPRA score further incorporates variables, such as percentage of positive biopsies and patient age, our results suggest that this system cannot preferentially show prognostic benefit over the 5-tier NCCN system. ROC curve analysis did not effectively discriminate between the two approaches, as the AUC values consistently remained around 0.6 or lower and did not reach statistical significance. Therefore, choosing between these two methods could be based on personal or institutional preferences.
In the absence of large-scale, randomized studies comparing curative approaches in the treatment of high-risk PCa, the combined approach using EBRT, HDR-BT boost, and ADT might offer some of the most favorable oncological outcomes for PCa patients. Kee et al. meta-analysis indicated a notable improvement in five-year b-PFS with brachytherapy boost, despite no survival advantage and a higher incidence of late-stage urinary and gastrointestinal toxicities [15]. Additionally, large retrospective analyses have suggested potential OS benefits over all other treatment regimens in favor of EBRT, HDR-BT, and ADT [16]. Despite this, our results show that very high-risk patients among an already high-risk cohort are two to three times more likely to relapse or die. This raises the question whether additional screening and/or treatment intensification should be considered for these patients.
The introduction of bi- and multiparametric MRI for the prostate along with image-guided biopsy techniques is set to significantly enhance the precision of diagnostic staging and the evaluation of biopsy core involvement [17], which in turn, could enhance the accuracy of the CAPRA score. To this end, the use of a combined scoring system with the 3-tier NCCN classification, PSA density, and mpMRI findings has been recently shown to improve prognostic accuracy [18]. Currently, these results are hindered by interpretation challenges as well as time- and resource-consuming equipment requirements, affecting its broader adoption [19].
Prostate-specific membrane antigen (PSMA) PET-CT has been shown to upstage high-risk and very high-risk PCa patients, challenging conventional imaging assessments [20, 21]. Xiang et al. also highlighted a PSMA-based nomogram’s effectiveness in predicting clinical outcomes, outperforming existing risk models [21]. Nonetheless, the risk of death from PCa remains low, even among higher risk cases [22], and most patients appear to benefit from local treatment combined with ADT [23]. It is therefore not yet clear if we should deny aggressive local therapy in patients with systemic disease found on PSMA PET-CT alone. Intensifying treatment through targeting the dominant intra-prostatic lesion (DIL) with advanced imaging techniques or employing novel antiandrogens, may be a preferable treatment method for these patients.
To this effect, the FLAME randomized phase III trial demonstrated how an additional simultaneous integrated focal boost up to 95 Gy to the intra-prostatic lesion visible on mpMRI can significantly improve biochemical disease-free survival (bDFS) [24]. At a 5-year follow-up, bDFS was 92% compared with 85% for standard treatment group, with no significant differences in late genitourinary and gastrointestinal toxicities. Using HDR-BT for partial dose escalation has been shown to be a safe and more effective way to achieve RT boost, without increasing treatment toxicity [16, 25, 26].
Regarding systemic treatment, the findings of STAMPEDE trial are changing practice and provide compelling evidence for administering 2 years of abiraterone acetate in conjunction with ADT and radiotherapy in very high-risk PCa [27]. The addition of abiraterone acetate highly improved OS for these patients [27].
This population greatly overlaps our very high-risk patients, whether defined by their CAPRA score or using the 5-tier NCCN risk stratification score, and further suggest the consideration of treatment intensification in this population. Other ongoing trials evaluating treatment intensification in the very high-risk population, such as the ATLAS study and PATRON phase III study (NCT04557501), should be completed soon, offering a more definitive answer to this question [28, 29].
Genomic-based risk stratification is also becoming increasingly popular [30-33]. To this effect, our institution is participating in The Prostate RNA Expression/Decipher To Individualize Concurrent Therapy with Radiation (Predict-RT) phase III trial that is estimated to be completed in 2025 (NCT04513717). The trial evaluates the use of genomic-based risk stratification to intensify or de-intensify the treatment of prostate cancer patients.
A similar study could be proposed for higher risk PCa patients, as evaluated by the CAPRA score or NCCN classification. The ongoing ENZARAD trial (NCT02446444) addresses this question without further re-classification of high-risk patients. The study is estimated to be completed by July 2024. One could hypothesize that the benefit of ART addition might be more pronounced in the very high-risk stratum of patients.
The concurrent DaSL-HiCaP study (NCT04136353) is a randomized phase 3 double-blind, placebo-controlled trial, in which darolutamide is added to androgen deprivation therapy as well as definitive or salvage radiation in very high-risk, clinically localized prostate cancer patients. The definition of very high-risk for this trial roughly corresponds to that of the NCCN. Therefore, this trial, which started in 2020, directly addresses the issue highlighted in the present study.
Strengths and limitations
This analysis has several strengths, such as a lengthy clinical follow-up, a large well-balanced and profiled cohort, and treatment provided by a skilled tertiary academic center. The major limitation of our analysis includes being a retrospective, single-institution study. The generalizability of our findings may be particularly limited to patients with higher pre-treatment PSA values, or those benefiting from different treatment regimes. Nonetheless, our findings align with existing literature with similar cohorts [34].
Conclusions
High-risk PCa patients classified according to the D’Amico and 3-tier NCCN classification systems benefit from further stratification using the CAPRA score or the newer 5-tier NCCN stratification system. Patients having a CAPRA score ≥ 6 or at very high-risk in the NCCN classification, are at greater risk of BCR, metastasis, and death. Concordantly, these patients could benefit from further intensification of their investigations and treatments, based on ongoing research. When compared with each other, none of these two tools demonstrated a better performance.