Purpose
Brachytherapy is an important treatment modality used for curative treatment of prostate cancer. For men with unfavorable intermediate-risk or high-risk prostate cancer, combination therapy with a brachytherapy boost and external beam radiation therapy (EBRT) has been shown to increase biochemical disease-free survival compared with EBRT alone [1]. High-dose-rate (HDR) brachytherapy is a promising approach for use in combination therapy based upon published results from several studies, which have demonstrated excellent disease control and favorable toxicity profiles with this approach [2-5]. Currently, there is an increasing interest in using magnetic resonance imaging (MRI) guidance to further refine HDR brachytherapy technique for prostate cancer [3, 6].
Historically, most prostate brachytherapy has involved delineation of organs at risk and target volumes as well as treatment planning on trans-rectal ultrasound (TRUS) and/or computed tomography (CT) imaging. Due to the multifocal nature of prostate cancer, brachytherapy has entailed targeting the entire gland with relatively uniform doses [7, 8]. While TRUS or CT provide adequate whole-gland visualization, multiparametric prostate MRI (mpMRI) allows for better soft tissue resolution and delineation of dominant intra-prostatic lesions (DILs) [9-11]. For both diagnostic workup and treatment guidance, mpMRIs are commonly obtained for men with intermediate- or high-risk prostate cancer [12, 13].
Literature investigating DILs has demonstrated that these lesions are clinically important and represent high-risk sites of disease [14]. Specifically, studies show that DILs are the highest risk sites for in-gland local recurrence following definitive RT and present the greatest risk site of harboring sites of higher Gleason score disease [15, 16]. The recently published FLAME trial demonstrated that dose escalation to DILs using IMRT results in improved biochemical disease control [17]. For these reasons, there is clinical interest in targeting and dose escalating DILs during brachytherapy, while also delivering standard treatment dose to the whole gland [18, 19].
To date, there have been several studies evaluating the feasibility of MRI-guided brachytherapy that have shown promising dosimetric outcomes. However, most studies investigating MRI-guided prostate brachytherapy have evaluated prostate monotherapy (HDR or low-dose-rate [LDR]), CT-based HDR planning, partial gland treatment, and salvage prostate treatment in the setting of local failure [20-25]. To date, the available prospective data suggests that using MRI guidance to dose intensify DILs is feasible, but the extent to which dose can be intensified has not yet been fully explored [23]. In this dosimetric study, we assessed the maximum achievable dose escalation to DILs while respecting organs at risk (OARs) objectives in the setting of combination therapy with TRUS-based HDR brachytherapy boost followed by EBRT. We hypothesized that implementing MRI guidance would allow significant DIL dose escalation, and we aimed to achieve a DIL D90 of greater than 150% while maintaining excellent OARs dosimetry.
Material and methods
Inclusion criteria
Our institution routinely treats patients with unfavorable intermediate-risk, high-risk, and very high-risk prostate cancer with androgen deprivation therapy (ADT) and combination HDR prostate brachytherapy (15 Gy in 1 fraction) followed by pelvic EBRT (45 Gy in 25 fractions). ADT consists of leuprolide prescribed for 6 months for unfavorable intermediate-risk group, and 18-24 months for high- or very high-risk group prostate cancer patients. For this retrospective dosimetric study, we identified consecutively treated patients who received combination therapy from 2013-2016. For inclusion in this study, patients had to have an available pre-treatment mpMRI with 1-3 visualized DILs. DILs were defined as PIRADS 4 or 5 lesions identified and marked on MRI by our institution’s diagnostic radiologists. A total of 24 eligible patients were found and included in this study. This study was performed with an approval from the Institutional Review Board for Health Sciences Research at our institution (approval number: IRB# 00000447).
Details of brachytherapy
High-dose-rate brachytherapy was performed according to institutional practice using TRUS-based treatment planning. A biplanar TRUS probe was applied (BK Medical; Peabody, MA, USA), and 14-20 stainless steel needles (Varian Medical Systems; Palo Alto, CA, USA) were placed using a modified peripheral needle arrangement. Treatment planning was performed using Vitesse software (Varian Medical Systems; Palo Alto, CA, USA), with contouring of prostate, clinical target volume (1-2 mm expansion of the prostate), and OARs, including the urethra, bladder, and rectum. A physician evaluated treatment plan with the knowledge of mpMRI but without the fusion of mpMRI to TRUS or DILs delineation. All patients received 15 Gy in a single fraction delivered using a VariSource iX HDR afterloader (Varian Medical Systems). After HDR brachytherapy, patients received either 45 Gy in 25 fractions to the prostate, seminal vesicles, and regional nodes, or 37.5 Gy in 15 fractions to the prostate and seminal vesicles. All 24 patients also received 6-24 months of ADT (leuprolide), which was delivered neoadjuvantly treatment (1-2 months), concurrently, and adjuvantly. Fiducial markers and polyethylene glycol prostate-rectal spacer (Boston Scientific; Marlborough, MA, USA) were inserted at the end of brachytherapy procedure, following HDR brachytherapy delivery in preparation for a EBRT course. Prostate-rectal spacer was placed following HDR brachytherapy delivery to maintain an adequate visualization of the prostate throughout the procedure and to avoid deformation of the prostate, which could affect dosimetry.
MRI acquisition and registration
Each patient had a diagnostic mpMRI prior to treatment. Nuclear imaging, including PSMA-PET (prostate-specific membrane antigen-ositron emission tomography) or fluciclovine F-18 PET, was not available. T1-weighted, T2-weighted, diffusion-weighted, and dynamic contrast-enhanced MRI sequences were acquired on a 3T-MRI scanner. MRI was performed at a median of 73 days prior to treatment. For purposes of this study, axial T2-MRI sequence was rigidly co-registered to TRUS post-needle placement acquired at the time of brachytherapy. Each rigid registration was reviewed and approved by both a radiation oncologist and a medical physicist. MRI was used to contour any visible DILs. Each patient had at least 1 DIL (range, 1-3, mean, 1.29, median, 1.26). DIL contours were transferred and fused to the TRUS-based planning system. Original TRUS-based prostate and OAR volumes were not altered. The included OARs were the urethra, bladder, and rectum. Brachytherapy needle placement locations from the original brachytherapy plan were not altered during experimental re-optimization.
Experimental re-optimization
The original treatment plans were experimentally re-optimized using BrachyVision v. 13.6 software (Varian Medical Systems; Palo Alto, CA, USA). The goal of experimental re-optimization was to maximally dose escalate the DILs while still respecting our institution’s standard OAR dose objectives. To be accepted, each re-optimized plan had to meet all pre-specified dosimetric targets or had to achieve dosimetry no worse than the original plan. Each re-optimized plan was deemed acceptable if it delivered a prescription dose of 15 Gy in 1 fraction and achieved all of the following objectives: prostate D90 > 100%, prostate V100 > 90%, urethra D10 < 118%, rectum V80 < 0.5 cc, and bladder D1cc < 75%. These dosimetric targets represent our institution’s standard dosimetric goals, and the same dosimetric objectives were used during the originally delivered treatment plans. Of note, not all of the original treatment plans met all of the specified dose objectives. If the experimentally re-optimized plan did not achieve all of these dosimetric objectives but the achieved dosimetry of re-optimized plan was no worse than the original plan, then the re-optimized plan was also deemed acceptable. Acceptable re-optimized plans were generated for all 24 patients. Dosimetric indices from the original and re-optimized plans were compared using a two-tailed paired t-test.
Results
Re-optimized plans for all 24 patients met the pre-specified acceptability criteria. Figure 1 shows the process of MRI DIL delineation, DIL co-registration on the originally delivered TRUS brachytherapy plan, and re-optimization to achieve DIL dose escalation. Figure 2 shows a dose volume histogram comparison of an originally delivered plan and a MRI re-optimized plan. Achieved dosimetry is reported in Table 1, and Supplemental Table 1 shows a full dosimetric report for each patient. The mean DIL D90 was significantly increased from 134% on the original plans to 154% on the re-optimized plans (p < 0.001). The mean urethra D10 and mean urethra V125 were significantly reduced from 123% to 117% and from 0.178% to 0.045%, respectively, on the re-optimized plans (p < 0.0001 and p = 0.007). The mean bladder D1cc was significantly reduced from 72% to 65% (p = 0.01). The mean rectum V80 and the mean rectum D2cc were unchanged (p = 0.27 and p = 0.33). On the re-optimized plans, the prostate D90 and V100 were reduced from 106% to 102% and 93% to 91%, respectively (p < 0.001 and p < 0.005). Decreased prostate coverage was considered acceptable because D90 > 100% and V100 > 90% were achieved.
Fig. 1
A) Dominant intraprostatic lesion (DIL) delineation on co-registered mpMRI. B) DIL contour superimposed on original brachytherapy plan. C) Experimentally reoptimized plan with DIL dose escalation
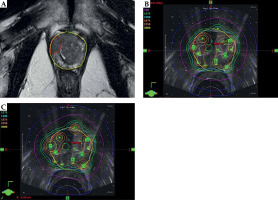
Fig. 2
Dose volume histogram comparison showing original plan dosimetry (triangles) and MRI reoptimized dosimetry (squares). GTV1 – dominant intraprostatic lesion
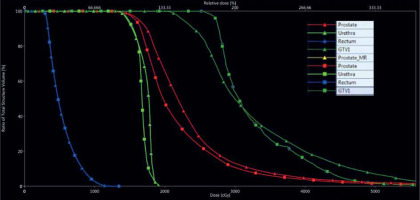
Table 1
Original plan and magnetic resonance imaging (MRI) re-optimized dosimetry for high-dose-rate (HDR) brachytherapy boost
Discussion
The present study suggests that MRI delineation and treatment planning using TRUS/MRI fusion is a feasible method of dose escalating DILs while maintaining excellent whole gland coverage and favorable OAR dosimetry. Prior studies have shown that DILs represent a high-risk site for local recurrence, so further escalating dose delivered to these lesions is a compelling strategy to reduce recurrence and improve clinical outcomes [15, 16]. Our observation in the current study, that dose escalation to DILs can be achieved with acceptable OARs doses and whole gland coverage, provides a foundation for an ongoing clinical trial developed at our institution.
When considering dose escalation as proposed here, it is critical to consider toxicity. Men with unfavorable intermediate- and high-risk prostate cancer already achieve good clinical outcomes with current standard treatment approaches. Indeed, one barrier to utilization of combined brachytherapy and EBRT is the high (18%) observed rate of grade 3 genitourinary toxicity reported in ASCENDE RT [1, 26]. While more modern series of combination HDR brachytherapy and intensity-modulated radiation therapy (IMRT) show far lower rates of genitourinary toxicity, many providers still do not offer combination therapy [2, 27, 28]. Additionally, one prospective clinical trial using HDR brachytherapy boost has demonstrated that DILs can be modestly dose escalated while maintaining a favorable toxicity profile [23]. Due to these toxicity-related concerns, we designed our approach in this study to minimize the risk of increasing toxicity. The experimental DIL dose escalation plans met standard dosimetric objectives for the urethra, bladder, and rectum, and in many cases, OAR dosimetry was improved despite dose escalating the DIL. Because these dosimetric objectives were achievable, we propose that DIL dose escalation would be unlikely to increase genitourinary or rectal toxicity.
An interesting finding on the dosimetric evaluation of the original brachytherapy plans was that DILs were already receiving a relatively high mean D90 of 134%. There are several possible explanations for why high DIL coverage was achieved even without MRI delineation and dose escalation. First, our institution uses a modified peripheral needle pattern that preferentially implants the peripheral zone. The peripheral zone is the highest risk location for DILs, so a needle implant that aggressively covers the peripheral zone may often escalate dose to DILs incidentally. Second, providers who performed the original brachytherapy treatment had prior knowledge of the patient’s biopsy results and the location of DILs seen on MRI. While no formal image registration or DIL targeting was performed, providers ‘cognitively boosted’ high-risk areas by ensuring needle placements in the regions of the prostate which were known to have high-grade disease on biopsy or DILs on MRI. While it is encouraging to see that high-risk DILs were well covered on the original plans, our results suggest that there is still room for further dose escalation to these areas.
When considering the results of this study, it is important to also consider the results of the FLAME trial [17]. The FLAME trial was a prospective randomized trial that treated whole prostate to 77 Gy in 35 fraction, while incorporating a simultaneous integrated boost to the DIL of up to 95 Gy. At 5-year follow-up, the DIL boost arm achieved superior biochemical disease-free survival (92% vs. 85%), and no significant increase in late toxicity. This is the first prospective data demonstrating that a DIL boost can improve clinical outcomes, and has stimulated growing interest in this topic. It is thought provoking to compare the equivalent doses achieved in the FLAME trial versus our present study. The FLAME trial assumed an α/β ratio of 1.2, and was designed to deliver an EQD2 of 81.8 Gy to the whole prostate and an EQD2 of up to 115.8 Gy to the DIL. The present study delivered 45 Gy in 25 fractions to the pelvis, and delivered a single-fraction HDR boost of 15 Gy to the whole prostate and 22.5 Gy to DILs. Using an α/β ratio of 1.2, this treatment achieved a cumulative EQD2 of 118.1 Gy to the whole prostate and an EQD2 of 208.8 Gy to DILs. While the FLAME trial represents a critical validation of the concept of a DIL boost, it is essential to note that brachytherapy boost continues to deliver a significantly higher effective dose both to the whole prostate and to DILs.
One limitation of this retrospective study is that the re-optimized plans used needle positions of the original brachytherapy plan. We acknowledge that placing needles directly within DILs improves dose escalation to DILs, and in our ongoing prospective institutional study, we do attempt to place needles centrally within the MRI-delineated DILs. However, for the purposes of this retrospective feasibility evaluation, we felt that needle placement for the experimentally re-optimized plans should not be changed. Altering needle placement would likely have improved our re-optimized plans, but retrospectively moving needle placements might have caused us to use needle positions that were not actually clinically achievable. By restricting our re-optimization to needle positions achieved during the original brachytherapy plan, we ensured that our optimizations represent plans that are feasible. We regard our results as a lower bound of what is dosimetrically achievable, and recognize that DIL directed needle placement should further improve dosimetric outcomes.
Finally, it is important to consider brachytherapy workflow when implementing techniques such as DIL dose escalation. We designed the proposed DIL dose escalation technique so that it can be quickly implemented within the confines of our current TRUS-based HDR prostate brachytherapy workflow. We estimate that the image registration, DIL contouring, and inverse planning would increase procedure time by no more than 15 minutes. Furthermore, the DIL contouring on MRI can be performed asynchronously prior to the brachytherapy procedure in order to avoid delays in the operating room. We chose to use MRI-based fusion and re-optimization in this study because the majority of modern data assessing DILs have used MRI, and MRIs are the most frequent advanced imaging modality available in our patient population. In recent years, there has been an increasing utilization of nuclear imaging, including PSMA-PET and fluciclovine F-18 PET in the initial diagnostic workup of prostate cancer, and these modalities are also being used to define DILs [29-31]. As nuclear imaging becomes increasingly available in our patient population, we hope to explore and implement similar image guidance and dose differentiation techniques using these imaging modalities.
Conclusions
Using MRI for delineation of DILs, we were able to re-optimize HDR brachytherapy plans to dose escalate DILs dose to a mean D90 of > 150% while maintaining favorable prostate coverage and OARs doses. Based on this finding, we estimate that a goal DIL D90 of > 150% (or 22.5 Gy) is an achievable dosimetric goal while meeting OAR dose constraints. We have used this analysis to inform our institution’s ongoing prospective clinical trial on MRI-guided prostate HDR brachytherapy.



