Purpose
The standard of care for the treatment of locally advanced cervical cancer (LACC) is concurrent cisplatin-based chemoradiotherapy (CRT) and image-guided brachytherapy (IGBT) [1,2,3,4]. In radiation therapy (RT), local control and normal tissue complication probability is related to dose of radiation delivered to the target volume and organs at risk (OARs), respectively [5]. Dosimetric characteristics of IGBT makes it possible to deliver an ablative dose to the target volume while sparing surrounding OARs due to the steep dose gradient, which is not safely feasible with external beam radiotherapy (EBRT) techniques alone [6,7]. Therefore, IGBT has recently formed an integral part of the RT and it has been introduced to improve treatment outcomes in LACC [8,9,10,11,12,13].
In the literature, Katz and Eifel [14], Perez et al. [15,16], and Viswanathan et al. [17] reported that local control and complication rates might be related with appropriate applicator selection and technical adequacy of the brachytherapy (BRT) implant. Therefore, selection of a suitable BRT applicator plays a crucial role to increase the treatment outcomes. In addition to applicator selection, treatment conditions like simulation or treatment protocol (rectum and bladder fullness), use of vaginal gauze packing (VGP), rectal spacer balloon (RSB), or rectal retractor (RR) significantly affect the quality of the treatment.
According to the International Commission on Radiation Units and Measurements (ICRU) report 89 [18], there are various types of intracavitary applicators used in cervical cancer BRT, and most of these systems are composed of two main components including intrauterine tandem and vaginal applicator. The very first roots of intracavitary BRT applicators can be traced to radium based low-dose-rate (LDR) applications [19]. With the advance in technology, these applicators have evolved over many decades and they have been modified for 137Cs and 60Co artificial radioactive isotopes. Nowadays, 192Ir-based high-dose-rate (HDR) systems and BRT applicators compatible with these systems are widely used in clinics [18]. Although there are various type of intracavitary BRT applicators as defined in ICRU report 89 [18], tandem-ovoids (TO) and tandem-ring (TR) are the two most common used applicators in LACC [20,21]. Additionally, intracavitary BRT application requires an appropriate retraction method to place away rectum and bladder from radiation sources. In this way, complication probability for rectum and bladder can be reduced. Typically, VGP, RSB, and RR are the most common retraction methods used in IGBT for cervical cancer [22,23,24].
In the present study, volume optimization-based inverse planning techniques were used for both applicator geometries (TO and TR) and retraction methods (VGP and RR). Treatment planning parameters were then compared in terms of dose volume histogram (DVH) parameters, dose volume indices, clinical target volumes (CTVs), and calculated dwell time in 10 Ci source activity. Although combined EBRT and BRT can result in significant urinary toxicity, urethra is not usually contoured as OAR, and there is a little existing data about urethral dose in the treatment of LACC. Therefore, in the present study, urethra was also contoured as an OAR and urethral dose was evaluated retrospectively. Additionally, upper, middle, and lower parts of the vaginal mucosa were contoured separately and doses were analyzed for each technique.
Material and methods
Patients
Ten patients with FIGO stage IB2 to IIIB cervical cancer, treated with concurrent CRT at our institute between July and November 2018 were included in this study. All patients’ FIGO stage distribution is presented in Table 1. All patients received 50.4 Gy whole pelvic EBRT in 28 fractions, using volumetric modulated arc therapy (VMAT) with Elekta Versa HD linear accelerator (Elekta AB, Stockholm, Sweden). Weekly cisplatin 40 mg/m2 concurrent with EBRT was administered to all patients. After EBRT treatment, 28 Gy HDR IGBT in 4 fractions were delivered to central disease with 192Ir sources using GammaMed Plus iX BRT unit (Varian Medical Systems, Palo Alto, CA). The study population was selected retrospectively among the patients who were topographically compatible with intracavitary BRT and treated with TO for one insertion and TR for another insertion. In this way, it was aimed to avoid patient-related differences like anatomical variations between two different patients.
Intracavitary BRT application
The intracavitary application was performed under conscious sedation in operating room conditions. Foley’s catheter was inserted to obtain reproducible bladder fillings at the time of computed tomography (CT) simulation and during BRT treatment. For both TO and TR applications, the same tandem geometry, 6 cm tandem length and 45 degree tandem angle, were used to minimize uncertainties due to differences in tandem angle and length. The most commonly used sizes of the ovoid for TO applications were 2.0-2.5 cm, and 5 mm build up cap was used in TR applicator. Anterior VGP was used to push the bladder and to stabilize applicator in both techniques. In TR applicator, RR was used as a retraction method and extra posterior VGP was not performed. In TO applicator, posterior VGP was carefully completed to displace the rectum away from the radioactive source, and at least 2-2.5 m packing gauze length was used for optimal packing.
Simulation and treatment planning
All patients underwent a CT scan using Toshiba Aquilion LB CT Simulator (Toshiba Medical Systems, Otowara, Japan) after every insertion with applicators in place. As a CT simulation protocol 100-120 kVp tube voltage, 300-350 mAs current value and 2 mm slice thickness were used. After simulation processes, CT images were transferred to BrachyVision treatment planning system version 8.9 (TPS) (Varian Medical Systems, Palo Alto, CA) via digital imaging and communication in medicine (DICOM) connection.
Target volume determination was performed according to the gynecologic examination, and magnetic resonance imaging (MRI) findings at diagnosis and just before BRT treatment were completed following the recommendations of the Group Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) [25,26]. According to the recommendations, high-risk (HR) and intermediate-risk (IR) CTVs were delineated. As for OARs, bladder, rectum, sigmoid, small bowel, and urethra were delineated [27], and vaginal mucosa including upper, middle and lower parts were contoured separately according to Westerveld et al.’s recommendations [28].
Treatment planning and dose calculation for the HDR BRT was performed using BrachyVision TPS. Volume optimization tool was used during the inverse optimization of the treatment plans, and similar optimization parameters were used to make it user-independent for both applicator geometries and retraction methods. Before starting the optimization, step size was set as 5 mm and the maximum active position range of the tandem for both TO and TR application was set as 7 cm from the tip of the tandem. For ovoids, the maximum active position range was set as 4 cm from the tip of the catheter and circular part was completely activated for ring applicator to mimic the standard loading pattern of manual optimization. Additionally, optimization parameters for inverse planning is presented in Table 2. As a clinical protocol, it was aimed to deliver at least 85 Gy (EBRT + BRT) equivalent dose in 2 Gy fractions (EQD2) to 90% of CTVHR volume (D90) and minimum EQD2 dose of 65 Gy to 90% of CTVIR volume (D90). For OARs, the total dose in D2cm3 were limited to 90 Gy for bladder and 75 Gy for rectum, sigmoid, and small bowel. To calculate the total dose of EBRT plus BRT plans, linear quadratic model was applied with a reference dose rate of 0.5 Gy/h, a half-time repair of 1.5 h, an α/β ratio of 10 Gy for CTVs, and 3 Gy for OARs.
Table 2
Optimization parameters for inverse planning
A total of 20 fractions corresponding to one fraction TO and one fraction TR-based treatment planning for each patient were analyzed to evaluate the dosimetric differences between two different applicator geometries and rectal retraction methods. Fractions included in the BRT treatment are presented in Table 1. In the analysis, DVH parameters of the CTVHR (D90 and D98), CTVIR (D90 and D98), bladder (D2cm3, D0.1cm3, and V7Gy), rectum (D2cm3, D0.1cm3, and V5Gy), sigmoid (D2cm3, D0.1cm3, and V5Gy), small bowel (D2cm3, D0.1cm3, and V5Gy), urethra (D2cm3, D1cm3, and D0.1cm3), and vaginal mucosa (D2cm3, D0.1cm3, V7Gy, and V10Gy) were noted for the comparison between TO with VGP and TR with RR applicators. Additionally, ICRU recto-vaginal point dose, vaginal reference point dose, differences in the target volumes, calculated total dwell times, weighting of the vaginal sources, and intrauterine sources in 10 Ci source activity per fraction were analyzed for both applicator geometries and rectal retraction methods. Moreover, reference isodose volumes including TVDref, TV1.5Dref, TV2.0Dref, VDref, V1.5Dref, V2.0Dref, V60Gy EQD2, and V85Gy EQD2 were reported to evaluate the conformity of the treatment planning. TVDref, TV1.5Dref, and TV2.0Dref were the volumes of CTVHR receiving dose greater than or equal to 100%, 150%, and 200% of the prescription dose, respectively. Similarly, VDref, V1.5Dref, and V2.0Dref were the total volume receiving dose greater than or equal to 100%, 150%, and 200% of the prescription dose in external body, respectively.
Statistical analysis
All data were recorded and analyzed on Statistical Package for Social Sciences (SPSS) software (version 20, IBM). Descriptive statistics (mean and standard deviation) were calculated and unpaired Student’s t-test was used to assess the relationship between the dosimetric values of two different applicators and retraction methods. P < 0.05 was considered to be statistical significant.
Results
Dose-volume histogram parameters for CTVs and OARs are presented in Table 3. D90 and D98 value for CTVHR and CTVIR were found to be statistically similar for both applicators. Although, there were no statistical differences in bladder (D2cm3 and D0.1cm3), sigmoid (D2cm3, D0.1cm3, and V5Gy), small bowel (D2cm3, D0.1cm3, and V5Gy), upper vaginal mucosa (D2cm3, D0.1cm3, and V10Gy), and urethra (D2cm3, D1cm3, and D0.1cm3), mean value of these parameters for all defined OARs were found lower in TR with RR than in TO with VGP (Table 3). Moreover, bladder (V7Gy), upper vaginal mucosa (V7Gy), middle and lower vaginal mucosa (D2cm3 and D0.1cm3) values were all found to be significantly lower for TR with RR than for TO with VGP (p < 0.045). In terms of rectal sparing, TR with RR was found to be significantly better than TO with VGP (p < 0.0001 for D2cm3, p < 0.000 for D0.1cm3, and p < 0.013 for V5Gy). Similarly, as presented in Table 4, ICRU recto-vaginal reference point dose were found to be statistically lower for TR with RR than for TO with VGP. However, there were no statistically significant differences in ICRU vaginal reference point doses for both applications (Table 4). In addition to DVH parameters, volumes of CTVs and total dwell times in 10 Ci source activities are shown in Table 5. CTVHR and CTVIR volumes contoured in TR-based applications were approximately 11% and 9% smaller than TO-based applications, respectively. The typical view of delineated CTVs and dose distributions of both applications are illustrated in Figures 1 and 2, respectively. When the total irradiation time per fraction was calculated, mean dwell time in 10 Ci source activity for TO and TR applicators were found as 386 s and 302.4 s, respectively (p < 0.0218); weighting of the vaginal and intrauterine sources for each patient are presented in Table 6. Additionally, TR with RR was found to be statistically superior than TO with VGP in terms of VDref, V60Gy EQD2 and V85Gy EQD2 as presented in Table 7.
Table 3
Comparison of DVH parameters for TO with VGP and TR with RR
| DVH parameters | TO (Mean ±SD) | TR (Mean ±SD) | ΔMean ± SD (TO-TR)* | p value |
|---|---|---|---|---|
| CTVHR D90 (cGy) | 706.2 ±4.5 | 710.6 ±5.5 | –4.4 ±6.4 | 0.0647 |
| CTVHR D98 (cGy) | 574.5 ±27.8 | 595.3 ±16.6 | –20.8 ± 34.8 | 0.058 |
| CTVIR D90 (cGy) | 510.8 ±32.2 | 497.3 ±37.9 | 13.5 ±38.8 | 0.402 |
| CTVIR D98 (cGy) | 398.4 ±28.4 | 384.6 ±44.8 | 13.8 ±53.5 | 0.422 |
| Rectum D2cm3 (cGy) | 493.7 ±60.0 | 357.4 ±62.9 | 136.3 ±40.2 | 0.0001 |
| Rectum D0.1cm3 (cGy) | 658.2 ±67.6 | 493.7 ±87.9 | 164.5 ±79.7 | 0.0002 |
| Rectum V5Gy (cm3) | 2.2 ±1.6 | 0.2 ±0.4 | 2.0 ±1.3 | 0.0013 |
| Bladder D2cm33 | 585.3 ±134.1 | 490.8 ±97.2 | 94.5 ±93.6 | 0.088 |
| Bladder D0.1cm3 (cGy) | 726.1 ±67.6 | 670.2 ±87.9 | 55.9 ±272.2 | 0.582 |
| Bladder V7Gy (cm3) | 1.0 ±0.9 | 0.3 ±0.5 | 0.7 ±1.0 | 0.035 |
| Small bowels D2cm3 (cGy) | 356.4 ±172.8 | 322.7 ±146.9 | 33.7 ±132.8 | 0.644 |
| Small bowels D0.1cm3 (cGy) | 498.2 ±217.9 | 443.5 ±215.9 | 54.7 ±170.0 | 0.580 |
| Small bowels V5Gy (cm3) | 1.6 ±2.5 | 0.6 ±0.9 | 1.0 ±2.0 | 0.206 |
| Sigmoid D2cm3 (cGy) | 323.9 ±193.9 | 265.1 ±134.3 | 58.8 ±169.5 | 0.441 |
| Sigmoid D0.1cm3 (cGy) | 420.3 ±251.5 | 316.6 ±179.7 | 103.7 ±276.0 | 0.303 |
| Sigmoid V5Gy (cm3) | 1.3 ±2.1 | 0.3 ±0.7 | 1.0 ±1.7 | 0.159 |
| Upper vaginal mucosa D2cm3 (cGy) | 798.7 ±158.5 | 656.5 ±208.6 | 142.2 ±173.7 | 0.103 |
| Upper vaginal mucosa D0.1cm3 (cGy) | 1217.0 ±425.1 | 1179.0 ±456.2 | 38.0 ±108.1 | 0.85 |
| Upper vaginal mucosa V7Gy (cm3) | 2.9 ±2.2 | 1.2 ±0.9 | 1.7 ±2.1 | 0.0318 |
| Upper vaginal mucosa V10Gy (cm3) | 0.6 ±0.8 | 0.4 ±0.4 | 0.2 ±0.9 | 0.409 |
| Middle vaginal mucosa D2cm3 (cGy) | 380.9 ±141.8 | 233.4 ±102.5 | 147.5 ±76.9 | 0.0158 |
| Middle vaginal mucosa D0.1cm3 (cGy) | 617.9 ±296.8 | 343.8 ±149.9 | 274.1 ±185.3 | 0.018 |
| Lower vaginal mucosa D2cm3 (cGy) | 147.7 ±59.5 | 97.8 ±30.3 | 49.9 ±43.1 | 0.0153 |
| Lower vaginal mucosa D0.1cm3 (cGy) | 196.0 ±104.6 | 119.6 ±40.2 | 76.4 ±78.0 | 0.045 |
| Urethra D2cm3 (cGy) | 50.8 ±50.0 | 31.1 ±34.8 | 19.7 ±27.2 | 0.32 |
| Urethra D1cm3 (cGy) | 144.3 ±53.2 | 95.2 ±68.8 | 49.1 ±5 3.8 | 0.0911 |
| Urethra D0.1cm3 (cGy) | 296.7 ±90.0 | 213.0 ±88.8 | 83.7 ±53.0 | 0.051 |
Table 4
ICRU recto-vaginal and vaginal reference point dose for both TO with VGP and TR with RR
| Planning parameters | TO (Mean ±SD) | TR (Mean ±SD) | ΔMean ±SD (TO-TR)* | p value |
|---|---|---|---|---|
| Recto-vaginal point dose (cGy) | 465.7 ±47.3 | 360.1 ±49.5 | 105.6 ±57.0 | 0.0001 |
| Vaginal point dose left (cGy) | 777.5 ±209.2 | 705.2 ±145.6 | 72.3 ±113.3 | 0.382 |
| Vaginal point dose right (cGy) | 745.4 ±131.1 | 725.5 ±142.9 | –19.9 ±233.4 | 0.749 |
Table 5
Target volumes and total dwell times in 10 Ci source activity for TO and TR applicators
| DVH parameters | TO (Mean ±SD) | TR (Mean ±SD) | ΔMean ±SD (TO-TR)* | p value |
|---|---|---|---|---|
| CTVHR volume (cm3) | 32.8 ±10.3 | 29.4 ±8.0 | 3.4 ±9.0 | 0.424 |
| CTVIR volume (cm3) | 77.4 ±30.1 | 70.8 ±30.5 | 6.6 ±13.9 | 0.633 |
| Total dwell time (s) | 386.0 ±80.6 | 302.4 ±67.7 | 83.6 ±65.9 | 0.0218 |
Fig. 1
A typical view of delineated CTVHR (red) and CTVIR (green) volume in coronal image section for A) TR and B) TO applicators on a same patient
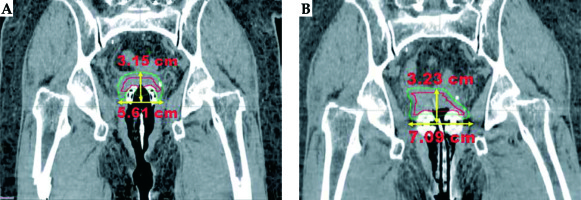
Fig. 2
Dose distributions in coronal and sagittal image sections using A) TR and B) TO applicators on the same patient
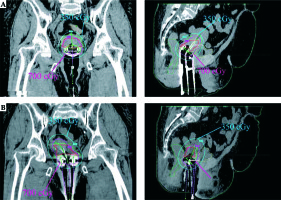
Table 6
Weighting of the vaginal and intrauterine sources in inverse optimization-based planning
Table 7
Reference dose volumes in CTVHR and external body for both TO with VGP and TR with RR
* ΔMean (TO-TR)* – mean value of the difference, **V1.5Dref and V2.0Dref are the volumes of external body receiving 150% and 200% of the prescription dose, respectively, TO – tandem-ovoids, TR – tandem-ring, VDref – volume receiving dose greater than or equal to the prescribed reference dose in external body, TVDref – volume of HR-CTV receiving dose greater than or equal to the prescribed reference dose, V1.5Dref – volumes of external body receiving 150% of the prescription dose, TV1.5Dref – volumes of HR-CTV receiving 150% of the prescription dose, V2.0Dref – volumes of external body receiving 200% of the prescription dose, TV2.0Dref – volumes of HR-CTV receiving 200% of the prescription dose, V60Gy EQD2 – isodose surface volume of 60 Gy equivalent dose in 2 Gy per fraction, V80Gy EQD2 – isodose surface volume of 85 Gy equivalent dose in 2 Gy per fraction
Discussion
The definitive treatment of LACC includes both pelvic EBRT with concomitant platinum-based chemotherapy and BRT boost to the central disease. TO and TR are the two most common intracavitary BRT applicators used in the treatment of LACC [1,2,3,4,18,20,21]. TO applicator is the variation of traditional Manchester, Fletcher, Henschke systems, and TR applicator is derived from the Stockholm system [18]. TR applicators have recently come into widespread use in IGBT of cervical cancer due to the easiness of application compared to TO applicators. Additionally, it has a predictable and fixed geometry [20,21,29]. All these physical properties of TR applicator make it more advantageous in clinical use. Although TR applicator can be used in all patient groups requiring intracavitary BRT, patients with non-bulky disease, superficial or obliterated vaginal fornix, or narrow vaginal cavity are the ideal patients’ group in cervical cancer [20,21,29]. In addition to applicator geometries, a retraction method play an important role to place away rectum from radiation source and to increase the conformity of the BRT treatment plan [22].
In the literature, the dosimetric comparison of different applicator geometries (TO and TR) and rectal retraction methods (RR, VGP, and tandem Foley balloon) have been studied. Ma et al. [20], Rangarajan [29], and Erickson et al. [30] reported higher rectal dose in TO compared with TR applicator. In the study performed by Rangarajan [29], it was pointed out that in addition to posterior VGP, RR was used in all TR applications and lower rectum dose in TR applications was attributed to the use of RR. Additionally, Kong et al. [22] compared three rectal retraction methods including RR blade, VGP, and a tandem Foley balloon. They discovered that RR was more advantageous in terms of rectal dose compared to other defined methods. In the present study, TR applicator with RR was also found to be statistically superior to TO applicator with posterior VGP in terms of rectal sparing.
In terms of bladder doses, Ma et al. [20] and Rangarajan [29] reported that there were no significant differences between TO and TR for D2cm3 value. Similarly, statistically significant difference was not found for the same DVH parameter in the present study. However, there was a slight trend towards increased D2cm3 value, with TO and V7Gy for bladder founded to be significantly higher in TO compared with TR-based treatment plans.
In addition to rectum and bladder dose, in the present study, upper, middle, lower vaginal mucosa, and urethral dose were evaluated. Although vaginal mucosa and urethra have been considered as radio-resistant organs, recent studies have reported a higher incidence of late vaginal toxicity (e.g. vaginal shortening and dyspareunia) and severe urethral toxicity (e.g. urethral necrosis) in the treatment of gynecological patients with combined treatment of EBRT and BRT [31,32]. However, so far, there is a little existing data about dose tolerance limits for vaginal mucosa and urethral dose in the treatment of LACC. To the best of our knowledge, this is the first comprehensive study to evaluate urethral dose and vaginal mucosa dose including upper, middle, and lower parts separately for both applicator geometries. Although, in the literature it was stated that vaginal mucosa dose might be higher in TR applicators compared with TO due to the smaller thickness of the build-up material [30,33,34], in the present study, vaginal mucosa dose was found lower in TR with RR than in TO with VGP. In fact, significant differences were demonstrated for upper vaginal mucosa (V7Gy), and middle and lower vaginal mucosa (D2cm3 and D0.1cm3) values. This can be attributed to the fact that total volume receiving dose greater than or equal to the prescribed reference dose in external body was found to be higher in TO than in TR applicator.
Ma et al. [20] and Levin et al. [21] also reported that contoured target volumes were smaller in TR compared to TO applicators. Similar differences were observed in the present study, and the differences in delineated volumes can be attributed to the geometric design of TO applicator, allowing for wider transverse displacement of the CTVHR and CTVIR volumes at the level of the ovoids, when compared with ring applicator. Additionally, in the study performed by Ma et al. [20] and Levin et al. [21], the dose prescription was performed according to reference point A as defined in ICRU 38 [35]. However, in the present study, the reference dose was prescribed to D90 value of CTVHR and CTVIR, as recommended in GEC-ESTRO guideline [26]. Additionally, the comparison of different applicator geometries and retraction methods in the same patient anatomy can be shown as one of the major advantage of the present study to minimize the effects of anatomical differences between two patient groups.
In terms of irradiation time, similar to Ma et al. [20] and Levin et al. [21], total dwell times for TR applicator were found lower than that for TO applicator. This difference could be attributed to the geometry of intravaginal component of the BRT applicators. According to our analysis, in TO applicator weighting of the intrauterine source was found higher than intravaginal source during the irradiation of the anterior part of target volume. In contrast to TO applicator, the weighting of the intravaginal source was found higher than intrauterine source in TR applicator during the irradiation of related part of the target volume. This could be related to the fact that the upper part of intravaginal component in TR applicator was so much closer to the defined region than that of both intravaginal and intrauterine component in TO applicator. Due to the closer source position to the target volume, total dwell times were also found lesser in TR applicator than that in TO applicator. Another noteworthy point is that in the present study, volume-based optimization method has been validated for both applicator geometries (TO with VGP and TR with RR) in defined conditions. Similarly, in the literature, Jamema et al. [36] and Kannan et al. [37] reported that inverse planning could offer good sparing of critical structures without compromising the target coverage compared to standard techniques, and so it could improve the quality of treatment plans in intracavitary BRT. Nevertheless, as stated by Chajon et al. [38], straightforward use of inverse optimization might generated significant heterogeneity in dwell times, and loading pattern of the source in inverse optimization could be completely different from the standard loading pattern. Therefore, inverse optimization protocols should be validated before implementation in clinical practice and the loading pattern of the source should be verified after each optimization, since it requires extra caution to test the dosimetrical relevance of new optimization techniques in intracavitary BRT modalities. In the present study, loading pattern of the intrauterine and intravaginal sources for all treatment plans were also validated using manual planning technique, and it was found almost similar for both manual planning (54.5-45.5% for TO and 40.1-59.9% for TR) and inverse optimization-based planning (53.0-47.0% for TO and 38.0-62.0% for TR).
This study has also some limitations that have to be pointed out. The first limitation is that in TR applicator, RR was used as a retraction method and extra packing procedure was not performed. However, in TO applicator, VGP was performed due to the fact that there was no rectal retractor integrated into the existing system. Therefore, in terms of rectal sparing, two different applicator geometries with different retraction methods including TO with VGP and TR with RR were analyzed together. Another limitation is that this study focused only on treatment planning parameters of different applicator geometries and retraction methods. However, the effectiveness of the systems or applicator geometries was not evaluated in terms of short- and long-term clinical outcomes. Therefore, clinical studies need to be carried out to evaluate the correlations between dosimetric and clinical parameters for defined applicator geometries and retraction methods.
Conclusions
The results showed that there were no statistical differences in D90 value of CTVHR and CTVIR. However, all DVH parameters for OARs in TR were found to be better than in TO applicators. In terms of rectum dose, TR with RR provides statistically better rectal sparing than TO with posterior VGP. Nevertheless, short- and long-term clinical impact of the dosimetric differences for two different applicator geometries and retraction methods needs to be evaluated further.



