Summary
Transapical implantation of artificial chordae on a beating heart under two- and three-dimensional transesophageal echocardiographic guidance with the NeoChord DS1000 device is a new minimally invasive surgical technique to treat degenerative mitral regurgitation. The current observation is the largest report of Polish experience assessing early results of transapical polytetrafluoroethylene chord implantation as the treatment of severe mitral regurgitation in 21 patients with posterior leaflet prolapse. A significant reduction of regurgitation grade, early restoration of normal functional status and favorable echocardiographic reverse remodeling were observed 6 months after the surgery. Mitral valve repair with the NeoChord DS1000 device is feasible in properly selected patients and may be considered as an alternative to conventional procedures.
Introduction
The most common cause of primary mitral regurgitation (MR) is degenerative mitral valve (MV) disease including mitral valve prolapse (MVP) [1]. Degeneration of the MV affects approximately 2% of the population and has a wide pathologic spectrum, from fibroelastic deficiency to Barlow’s disease [1, 2]. An isolated MV prolapse or flail most commonly affects the middle scallop of the posterior leaflet (P2) with significant MR as the main consequence of leaflet malcoaptation [1, 2].
Echocardiography is a key diagnostic method used to diagnose MV disease, differentiate degenerative MR from functional MR and identify its consequences [1–3]. Valve repair remains the preferred surgical treatment for severe MR due to MVP [3, 4].
Traditionally, posterior leaflet mitral valve repairs have been done with large leaflet resections with annular plication [5]. Then implantation of artificial chordae from polytetrafluoroethylene was popularized by Tirone David and Friedrich Mohr [6, 7]. Over time many surgeons transitioned from leaflet resections to chordal reconstructions [8].
Since patients are being referred for surgery earlier, the need for reductive annuloplasty in every patient becomes questionable [9].
Additionally, since patients referred for MV repair are older, there is growing interest in techniques not requiring use of cardiopulmonary bypass.
In recent years, minimally invasive transapical techniques of MV surgery have been increasingly used [10]. Transapical implantation of artificial polytetrafluoroethylene chordae on a beating heart with the NeoChord DS1000 system using two-dimensional (2D) and three-dimensional (3D) transesophageal echocardiography (TEE) guidance is a new minimally invasive surgical technique to treat degenerative MR [11].
The NeoChord DS1000 system received the CE mark in 2012 and currently clinical experience is accumulating. This technique facilitates implantation of neochords fixed not to papillary muscles, but the apical area without complementary annuloplasty. Chordal length adjustment is done on the beating heart [11]. There are no data indicating whether this technique produces durable results comparable with conventional surgery.
A close and regular follow-up using transthoracic echocardiography (TTE) is recommended to evaluate the results of treatment with the NeoChord device. There are observations indicating safety and effectiveness of this technique and left ventricular (LV) and left atrial (LA) reverse remodeling in operated patients [12, 13].
Aim
The goal of this single-center observational study was to evaluate 6-month results of MV repair with the NeoChord DS1000 device in the first group of consecutive patients operated on in Poland using echocardiography.
Material and methods
During the period from October 2014 to August 2017, we performed echocardiographic examinations in 63 patients due to MVP with significant MR as potential candidates for the NeoChord procedure.
Inclusion criteria were: severe MR, posterior leaflet prolapse or flail, single eccentric MR jet due to prolapse/flail, good potential for coaptation (at least 20% of tissue overlap – leaflets vs. annulus), healthy, long anterior leaflet (longer than 70% of antero-posterior end systolic annulus diameter), technical possibility to place chords.
Exclusion criteria included: severe LV enlargement, no tissue overlap (leaflet to annulus ratio of less than 1), functional MR, multiple jets in different areas, endocarditis, significant leaflet and annulus calcifications, bileaflet prolapse, prolapse/flail of the commissural regions (technical difficulty to place chords and imaging limitations), leaflet perforation.
Eventually, 21 patients who had symptomatic severe MR due to posterior leaflet prolapse or flail (81% male; mean age: 60.7 ±12.7 years) were qualified for MV repair with the NeoChord DS1000 system (NeoChord, Inc., St. Louis Park, MN, USA) based on complete 2D TTE and 2D/3D TEE images. Detailed clinical and echocardiographic evaluation was performed at the time of qualification. All patients gave written informed consent for TEE and valve surgery.
We obtained preoperative TTE and TEE and follow-up TTE images using the iE 33 (Philips Medical Systems, Andover, Massachusetts) and intraoperative TEE views using the EPIQ 7 system (Phillips, Eindhoven, NL). The transesophageal X7-2t Live 3D xMATRIX array transducer was used.
Standardized TTE and TEE examinations were performed and interpreted by an experienced physician in patients placed in the left lateral decubitus position, with constant monitoring of one ECG lead according to the recommendations of the European Association of Cardiovascular Imaging (EACVI) [14].
Before the surgery, we assessed the mechanism and severity of MR, the LV and LA morphology and secondary changes in the right heart according to the current recommendations [1, 3].
We diagnosed severe MR based on qualitative and semiquantitative criteria including the vena contracta width (VC) ≥ 7 mm, a large central or eccentric jet reaching the posterior wall of the LA, a large convergence zone and a dense signal of MR in continuous wave Doppler.
The quantitative parameters such as an effective regurgitant orifice area (ERO) and regurgitant volume were evaluated with ERO ≥ 0.4 cm2 and volume ≥ 60 ml, which defined severe MR. Additional criteria of severe MR included systolic flow reversal in pulmonary veins, a dominant E wave in mitral inflow and time–velocity integral ratio: TVI mitral/TVI aortic > 1.4 [1, 3]. The diagnosis of severe MR was based on aggregate analysis of the above criteria.
Dimensions and volumes of the left cardiac chambers, such as the LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD), LV end-diastolic and end-systolic volume (LVEDV, LVESV), LA anteroposterior (a-p) diameter, LA volume (LAV), LA volume index (LAVI) and mitral annulus a-p size were measured. Maximal velocity of the early wave (E) in mitral inflow, and maximal diastolic myocardial velocities of the lateral and septal part of the mitral annulus (E’) were registered. Left ventricular ejection fraction (LVEF) was evaluated with the modified biplane Simpson’s method [14].
Additionally, tricuspid regurgitation peak gradient (TRPG) was measured and right ventricular systolic pressure (RVSP) was calculated and tricuspid annulus peak systolic excursion (TAPSE) was measured.
We distinguished three groups of patients: with an isolated central posterior leaflet prolapse or flail (type A) (Figure 1), with posterior multisegment disease/flail (type B) and with posterior/paracommisural disease (type C) [13, 15].
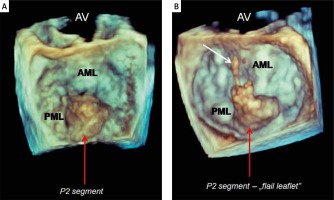
Figure 1
Preoperative three-dimensional transesophageal echocardiography; “surgeon’s view” of the mitral valve. An isolated posterior leaflet prolapse – P2 (red arrows). A – Prolapse of middle scallop without broken chords; B – “flail leaflet” – broken native primary chord to the posterior middle scallop (white arrow)
AV – aortic valve, AML – anterior mitral leaflet, PML – posterior mitral leaflet.
Mitral valve repair using the NeoChord device was performed under general anaesthesia with full hemodynamic monitoring. Thorax was entered through left minithoractomy lateral to the apex. The left minithoracotomy was the place of access. A surgeon introduced the NeoChord system into the LV cavity through a small incision under 2D/3D echocardiographic guidance. Intraoperative TEE images were visible for the surgeon on an additional dedicated monitor. During the whole procedure, 2D TEE multi-plane imaging (X-plane) and 3D TEE, the so-called “surgeon’s view”, were used interchangeably (Figure 2). Echocardiographic guidance included insertion of the NeoChord device, posterior leaflet grasping and capture, correct tension (length adjustment) of implanted neochords and an assessment of the final result. While the tip of the device was positioned at the level of the MV the posterior leaflet prolapsing segment was grasped and after fiber optic confirmation of leaflet capture, the leaflet was punctured with a needle. Then a loop and two ends of the NeoChord were pulled out from the LV, the leaflet was released and the operator removed the device from the cavity. Knots with the neochordae were tied on the leaflet and their free ends were secured on the muscle of the LV. Details of the NeoChord procedure are described in a previous publication from 2017 [11]. The procedures were performed by one surgeon and by the same echocardiography specialist.
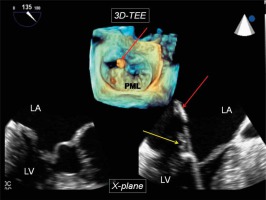
Figure 2
Echocardiographic guidance of the NeoChord procedure. Upper panel (three-dimensional imaging) – tip of the device visible in the left atrium during posterior leaflet grasping (red arrow); bottom panel (simultaneously visible X-plane imaging) – tip of the device in the left atrium (red arrow) and posterior leaflet tissue visible between jaws of the device (yellow arrow)
LA – left atrium, LV – left ventricle, PML – posterior mitral leaflet.
We evaluated early 6-month echocardiographic results including MR grade and parameters of LV and LA geometry and function and compared them with the baseline data obtained before the NeoChord procedure.
Postoperative MR was defined as trivial (“trace”) in cases of a very small, short jet, just above the valve, without a convergence zone, which was similar to physiological findings. We recognized mild MR in cases with the vena contracta width < 3 mm, the presence of a longer, but narrow jet (reaching up to half or 3/4 of the left atrium), systolic dominance in pulmonary vein inflow, A wave dominant in mitral inflow, no or a small convergence zone, and ERO < 0.2 cm2 and volume < 30 ml. Severe MR was defined according to the abovementioned criteria; moderate MR was identified when its parameters were more pronounced than in the mild MR but did not meet the criteria of severe MR.
Statistical analysis
Descriptive statistics for quantitative data were presented as means ± standard deviations. Qualitative data were shown as counts and percentages. Within-group comparisons for quantitative data were performed using the repeated measures t-test. In between-subject comparisons the Mann-Whitney test was used, due to the small sample size and large disparities in the group size. All reported results are based on two-sided statistical tests. Effects were considered significant at p-value less than 0.05. The statistical analyses were performed using R software (R Core Team, 2017), ver. 3.4.2.
Results
Baseline characteristics
Clinical and basic echocardiographic characteristics of 21 treated patients (17 male), aged 60.7 ±12.7 years, are presented in Table I. Twelve of the operated patients with severe MR had well-controlled hypertension and 5 patients had a history of paroxysmal or persistent atrial fibrillation. None of them had coronary artery disease, diabetes or chronic kidney disease. The majority of patients qualified for MV surgery were in NYHA class II. Preserved LV systolic function was found in 95% of the subjects. In most patients, hyperkinetic LV contractility was observed. Calculated RVSP was below 50 mm Hg in 81% of cases. There were 12 (57.1%) patients with type A MVP, 8 (38.1%) patients with type B and 1 (4.8%) patient with type C. This type C patient presented posterior leaflet prolapse in the region of the medial scallop (P3) close to the posteromedial commissure. A flail leaflet was present in 12 patients. The median number of implanted chords was 3 (2–6). Two patients received 2 chords, 14 patients 3 chords, 3 patients 4 chords, 1 patient 5 chords and 1 patient 6 chords.
Table I
Basic clinical and echocardiographic characteristics of patients qualified for mitral valve repair with the NeoChord DS1000 device
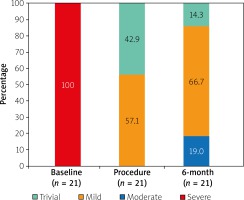
We achieved procedural success defined by the placement of at least 2 neochords with a significant reduction of MR, which means a decrease of the regurgitation from severe to trace/mild (defined according to the abovementioned criteria) in all patients (Figure 3). Very small residual MR called “trace” was found in 9 (42.9%) patients and mild MR in 12 (57.1%) patients. There were no postoperative complications and none of the patients required conversion to open mitral surgery.
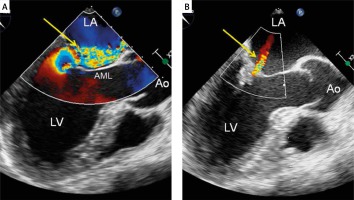
Figure 3
Mitral regurgitation evaluation using two-dimensional transesophageal echocardiography with color Doppler. A – Severe mitral regurgitation before the procedure – a wide eccentric jet directed to anterior mitral leaflet (arrow); B – trivial mitral regurgitation (very small central jet) as a final result of the NeoChord procedure (arrow)
Ao – aorta, AML – anterior mitral leaflet, LA – left atrium, LV – left ventricle.
Six-month data
At the 6-month follow-up all 21 patients markedly improved and all of them were in NYHA class I. Control echocardiography showed good function of MV in the majority of patients. In 17 (81%) studied patients nonsignificant MR (trivial in 3 (14.3%) patients, mild in 14 (66.7%) patients) was detected, while in 4 patients (19.0%) moderate MR was revealed (Figure 4). Neochordal dehiscence was not found in any patient. All 4 patients with a moderate MR had initially “flail leaflet” with at least 2 native chords broken (anatomical type A (3) and B (1)). Moreover, one of them had a dilated mitral annulus (46 mm) and LV cavity (70 mm). Three of them presented massive preoperative MR. Prior to the surgery, these 4 patients had a higher value of LVESD, LVESV and diastolic MV annulus diameter compared to the remaining subjects: (44 (36–57 mm) vs. 36 (25–46 mm), p = 0.039; 76.5 (59–166 ml) vs. 55 (20–84 ml), p = 0.043 and 41 (39–50 mm) vs. 38.5 (34–41 mm), p = 0.016, respectively).
Figure 4
Mitral regurgitation grades at baseline, at the end of the procedure and at the 6-month follow-up
Six months after the surgery, left-sided heart reverse remodeling caused by significant reduction of MR was observed. Left atrial a-p dimension, LAV and LAVI decreased significantly. Similarly, parameters of the left ventricle, such as LVEDD, LVESD, LVEDV, and LVESV, were significantly reduced. We noted a significant decrease in both systolic and diastolic mitral annulus dimensions. No hyperkinetic LV contractility was observed. The mean value of mitral inflow E wave velocity and maximal velocity E’ of the lateral part of the mitral annulus decreased significantly. Additionally, the mean values of TRPG and calculated RVSP were lower than before the procedure. There were no differences between mean values of the LV EF, TAPSE and maximal velocity E’ of the septal mitral annulus. Preoperative and postoperative echocardiographic data are shown in Table II.
Table II
Comparison of echocardiographic parameters before and 6 months after mitral valve repair with NeoChord DS1000 device in 21 patients
[i] Data are expressed as means and standard deviations. a-p – antero-posterior, D – diastole, E – maximal velocity of the early wave in mitral inflow, E’ – maximal diastolic velocities of the lateral and septal part of the mitral annulus, LA – left atrium, LAV – left atrial volume, LAVI – left atrial volume index, LVEDD – left ventricular end diastolic diameter, LVEDV – left ventricular end diastolic volume, LVESD – left ventricular end systolic diameter, LVESV – left ventricular end systolic volume, LVEF – left ventricular ejection fraction, MV – mitral valve, RVSP – right ventricular systolic pressure, S – systole, TAPSE – tricuspid annulus peak systolic excursion, TRPG – tricuspid regurgitation peak gradient.
Discussion
Mitral regurgitation is increasingly prevalent in Europe [3]. Following degenerative aortic stenosis MR is the second most frequent valve disease requiring surgery [3, 16]. Repair success depends on the MV morphology and surgical expertise [4].
There are several surgical techniques of MV repair used in patients with isolated posterior leaflet prolapse. In recent years a lot of surgeons have transitioned from quadrangular or triangular leaflet resections toward artificial chordae implantation. Leaflet preservation techniques result in better posterior leaflet motion as well as longer coaptation length [17]. Good leaflet coaptation can be achieved without reductive annuloplasty in approximately 20% of patients [9].
A lot of patients referred for MV repair are old and with significant comorbidities. Therefore, there is a clinical need for less invasive techniques without cardiopulmonary bypass and cross-clamping of the aorta dedicated to the repair of MVP, especially caused by posterior leaflet prolapse.
Echo-guided transapical implantation of artificial chordae on a beating heart with the NeoChord DS1000 system is a novel technique to treat degenerative MR, which has been the first transapical technique approved for MV repair in patients with MR due to prolapse or flail leaflet [10, 11, 13].
Initial experience with the NeoChord DS1000 system was presented by Seeburger et al. in 2010 [18]. According to the current report by Colli et al., which summarizes multicenter clinical experience, this technique of MV repair is feasible, safe and results in a good clinical outcome. A significant reduction in MR and an improvement of NYHA functional class has been achieved in the majority of patients one year after the surgery [13].
This type of surgical treatment is a promising therapeutic option for patients with MV posterior leaflet prolapse or flail [12, 13, 15]. Anatomical criteria, based on a preoperative 3D-TEE assessment, are of importance for the NeoChord procedure suitability [13].
Patients with an isolated central posterior leaflet prolapse/flail (P2) seem to be most suitable for this procedure (type A), patients with posterior multisegment disease/flail are acceptable (type B) and those with anterior leaflet or bileaflet MVP, with paracommissural disease or with MV calcifications are classified as a challenge (type C) [13].
Colli et al. compared outcomes according to the anatomical classification, and revealed a significant difference in the primary end point at 6 months among the anatomical groups type A, B and C [13].
Thus, a key role of 2D/3D echocardiographic evaluation of MV in a qualification process is unquestionable [19]. Presence of a large eccentric MR jet and sufficient leaflet tissue needed to achieve good coaptation should be confirmed by echocardiography before valve repair. The special role of 3D-TEE was emphasized by Demetrio et al., who have described the use of TEE before and during the NeoChord procedure in detail [20].
Our initial experience in the first 2 patients with posterior mitral leaflet prolapse treated with the NeoChord system was described in 2017 [11].
To our knowledge, the current echocardiographic observation is the largest report of Polish experience assessing 6 months of results of transapical polytetrafluoroethylene chord implantation as the treatment of MR due to mitral valve prolapse.
Our results revealed a full functional recovery in all treated patients. All 21 patients after 6 months presented functional NYHA class I. Of note, complete procedural success was observed in 100% of patients. At the 6-month follow-up a significant reduction of MR to trivial and mild was present in the majority of them (81%). There was no case with recurrent severe MR during this period. We observed a significant improvement in the left-sided heart geometry, expressed as a reduction in size and volume of the LV and LA. Similar results were presented by Rucinskas et al., who achieved procedural success in 92% of a group of 13 patients; at the 6-month follow-up they observed mild MR in 85% patients, and LV and LA reverse remodeling [12].
In the NeoChord Independent International Registry, Colli et al. reported postoperative results of 213 patients managed in 7 European centers. Acute procedural success was achieved in 96.7% of patients. After a 6-month follow-up of 198 patients, MR was absent/trace or mild in 77.3%, moderate in approximately 16% and severe only in 7% of patients. The percentage did not differ significantly at 1-year follow-up in the group of 191 patients [13].
As we expected, removal of the prolapsing segment and significant reduction of MR volume resulted in normalization of the mitral inflow pattern due to E wave decrease and slower movement of MV lateral annulus. We did not observe hyperkinetic LV contractility in any of the patients. Additionally, TRPG and calculated RVSP values were significantly lower than before the treatment. Importantly, a significant reduction in mitral annulus dimensions was also found.
Five-year follow-up results obtained by Kiefer et al. in 3 out of 6 patients treated with the NeoChord system in a German center were recently published. A good treatment effect was maintained for a 5-year period. Nonsignificant MR and no LV or MV annulus dilatation were observed [21].
It should be emphasized that an impact of proper patient selection on obtained results is inevitable. In our group, the 3 cases of moderate residual MR occurred in the first half of the operated patients, and in the second half there was only one case of moderate MR.
Study limitations
This is a single-center non-randomized observational study which included a relatively small number of consecutive patients treated with the NeoChord DS1000 device. An important limitation is a short follow-up of only 6 months.
An echocardiographic limitation may be a difficult quantitative assessment of MR due to complex and eccentric jets. Therefore, despite promising positive early experience, further investigation with a longer-term follow-up and a direct comparison with another mitral valve surgery is necessary.
Conclusions
Our data indicate that MV repair with the NeoChord DS1000 device resulted in restoration of normal functional status and favorable echocardiographic reverse remodeling 6 months after the surgery. In contrast to conventional cardiac surgery, the use of cardiopulmonary bypass, cardioplegia, and open heart surgery is not required.
Importantly, transapical implantations of neochords do not exclude the possibility of performing a classic MV surgery in the future.
However, in order to establish definitive safety and efficacy of this novel technique, a long-term follow-up is needed.








