Introduction
Diabetic non-healing wound (DNHW) refers to a situation where wound healing is hindered, slow, or ineffective due to various factors such as long-term hyperglycaemia, microcirculation disorders, neuropathy, and immune dysfunction. Its main clinical characteristics are slow healing, increased risk of infection, scar formation, etc. [1, 2], and its incidence rate is high. According to the data survey of the National Institutes of Health (NIH) in the United States [3], about 15–25% of diabetes patients will have wounds that are difficult to heal. With the increase of diabetes patients worldwide, the prevalence of DNHW is also gradually rising. For DNHW, currently, clinical methods such as controlling blood sugar levels, cleaning and wetting of wounds, biological therapy, and wound debridement are commonly used to treat patients, in order to reduce inflammatory reactions and promote wound repair [4].
With the in-depth research on DNHW, some scholars [5, 6] proposed that there is a close relationship between the disease and the immune microenvironment. When the immune system of diabetes patients is affected, it will lead to the imbalance of the immune microenvironment in the process of wound healing, thus affecting the effect of wound healing. As two proteins closely related to the immune microenvironment, α-smooth muscle actin (α-SMA) and collagen I (COL-1) play important roles in wound healing. Among them, α-SMA is a smooth muscle actin that is mainly present in smooth muscle cells and plays a role in granulation tissue formation during wound healing. The cytokines and growth factors in the immune microenvironment can promote the differentiation of fibroblasts into myofibroblasts, manifested by the increased expression of α-SMA. These transformed cells can participate in the important processes of wound contraction and granulation tissue formation. COL-1 is type I collagen, which is one of the most important types of collagen in wound repair. COL-1 plays a supporting and stabilizing role in the wound healing process. Under the regulation of the immune microenvironment, fibroblasts can synthesize and secrete COL-1, promoting collagen synthesis and deposition on the wound surface, and helping to form structurally intact tissues. Therefore, regulating the levels of α-SMA and COL-1 can be used to improve the wound immune microenvironment and promote wound healing in DNHW.
The development of modern society has led to an increasing level of medical technology in China, and the selection of treatment methods for DNHW has become increasingly diverse. Astragaloside (AST) is a Chinese herbal extract derived from Astragalus membranaceus. It mainly contains flavonoids (FL), polysaccharides (PS), amino acids (AA), and other components [7]. Among them, flavonoids are the main active components of AST, including flavonoid glycosides, isoflavone glycosides, etc. These compounds are considered to have antioxidant, anti-inflammatory, immune regulatory, anti-tumour and other biological activities. Several studies [8, 9] have shown that AST may promote the repair of diabetes wounds through multiple ways. Firstly, it can regulate inflammatory responses, suppress excessive inflammation, alleviate inflammatory damage, and provide favourable conditions for wound repair. Secondly, AST can also promote angiogenesis and repair around the wound, increase blood flow supply, promote wound healing, and may affect the migration and function of immune cells, enhance the immune response of the wound, and help combat infection. Although AST has shown its potential role in repairing diabetes wounds in the laboratory, its clinical application still needs further research and verification.
Aim
This article conducts in-depth research on the therapeutic effect of AST on DNHW through rat experiments, revealing its mechanism of action in wound healing, and providing theoretical basis for the development of new drugs and wound treatment. The report is as follows.
Material and methods
Experimental animals
Ninety adult male Wistar rats with SPF were observed and divided into three groups using a random number table method. A spreadsheet was drawn based on the rat numbers, and random non-repeated sampling was conducted using a computer logarithmic table with rat numbers in a ratio of 1 : 1 : 1. One group was a blank group (BG), one group was a control group (CG), and the other group was an observation group (OG). The rats were sourced from SPF grade rats from Shanghai Yishang Biotechnology Co., Ltd. The experimental animal use license number was SYXK (Shanghai) 2022-0029. The rats age in BG was 11–12 weeks, with an average of 11.47 ±0.40 weeks and a weight of 210–250 g, with an average of 220.76 ±11.25 g. The rats age in CG was 11–12 weeks, with an average of 11.53 ±0.44 weeks and a weight of 215–254 g, with an average of 223.16 ±10.99 g. The rats age in OG was 11–12 weeks, with an average of 11.61 ±0.81 weeks and a weight of 217–249 g, with an average of 230.24 ±13.20 g. The general data of the three groups of rats are consistent (p > 0.05), and this study has been approved by the ethics committee of our hospital before proceeding.
Reagents and instruments
(a) Sano Blood Glucometer: Sano Biosensing Co., Ltd., 2013 Hunan Food and Drug Supervision Equipment Qualification No. 2400102. San Nuo Blood Glucose Test Paper: An Yi Blood Glucose Meter Blood Glucose Test Paper, San Nuo Biosensing Co., Ltd., Hunan Equipment Qualification 20162400156, 50 pieces. (b) Physiological saline: Guangdong Huankai Microbial Technology Co., Ltd., product number CP0630225 ml*10 bags/box. (c) AST: High quality dried roots of Astragalus membranaceus were used as raw material. The roots of Astragalus membranaceus were crushed into fine particles, and AST was extracted using a suitable ethanol solvent and conventional extraction method. The extracted solution was filtered to remove solid residues and impurities. Then, the extraction solution was concentrated to a certain extent to concentrate AST, and cooling crystallization was used to further improve the purity of AST. After obtaining the crystal of AST, drying was carried out to remove the remaining solvent and make it a dry AST powder. (d) Improved Masson trichrome staining reagent kit: Beijing Solaybao Technology Co., Ltd., G1346-8*50 ml. (e) 4% paraformaldehyde tissue fixative: Shanghai Shangbao Biotechnology Co., Ltd., product number R22106500 ml. (f) Hematoxylin eosin (HE) staining kit: Shanghai Gefan Biotechnology Co., Ltd., product number M020A, 100 ml. (g) Rabbit derived α-SMA polyclonal antibody: Shanghai Yubo Biotechnology Co., Ltd., product number y0002a. (h) Rabbit derived COL-1 polyclonal antibody: Abnova, product number PAB17205. (i) Streptozocin (STZ): Shanghai Yuanye Biotechnology Co., Ltd., product number S17049. (j) 4℃ refrigerator: Thermo Fisher, PLR-386. (k) Mass spectrometry flow meter: Fluidigm, PLR-386, USA. (l) Pathological slicing machine: Leica, Germany, HistoCore Multicut. (m) Automatic dehydrator: Leica, Germany, ASP300S. (n) Paraffin embedding machine: Leica, Germany, HistoCore Arcadia H. (o) Bole ChemiDoc MP WB fluorescence imaging analyser: Bio Rad, Chemi-Doc MP. (p) Thermostatic incubator: Shanghai Fuma Company, DPX.9082.
Experimental Methods
Construction of the diabetes model
(a) Dietary management: Three groups of experimental rats were fed a high-fat and high-sugar diet, such as a high-fat or high-sugar diet, to induce weight gain and insulin resistance. (b) Induction of diabetes: multiple intravenous injections of STZ caused insulin deficiency and insulin resistance in rats, and induced diabetes. (c) Blood glucose monitoring: The fasting blood glucose levels of rats were monitored within 3 days after injection of STZ. If the fasting blood glucose of rats continued to rise and > 11.1 mmol/l, it indicated that the model construction was successful, and secondary construction was carried out on experimental rats that did not meet the standards.
Wound preparation
(a) Anaesthesia: Experimental rats were anesthetized with isoflurane to ensure painlessness and discomfort. (b) Disinfection: Before preparing the wound, the wound area of rats was thoroughly disinfected to reduce the risk of infection. (c) Localization: The dorsal area of the rat was used as the location of the wound. (d) Incision: A surgical knife or shaver was used to remove hair from the wound area, and a 2 cm circular incision was made in the wound area. During this period, attention should be paid to controlling of bleeding, which can be controlled using haemostatic agents or electrocoagulation.
Administration
BG: The wound was shaved only. CG: After shaving, the wound was covered with saline gauze and fixed with the adhesive tape. The saline gauze was replaced every 3 days. OG: After shaving the wound, AST powder was used to evenly cover the wound, covered with two layers of dry disinfectant gauze, and fixed with the adhesive tape. Replacement was performed every 3 days.
Observed indicators
Wound healing: The observation time was 1 day, 7 days, and 14 days after the formation of the wound. Image processing software ImageJ was used to analyse and process the wound images of three groups of experimental rats. Based on the Region of Interest (ROI) created, the wound area was obtained. Wound healing area = Starting wound area – Current wound area.
α-SMA and COL-1 levels: The observation time was 1 day, 7 days, and 14 days after the formation of the wound. The detection method was as follows: (a) tissue slices were prepared from wounds or other tissues and fixed with 10% concentration of formalin. Then the fixed tissue was cut into multiple small pieces. After embedding it with hot paraffin, the embedded tissue was cut into multiple thin slices. 3–5 complete slices were selected, dried in a 60°C constant temperature oven for 60 min, and fixed on glass slides. (b) By incubating the slices with specific α-SMA or COL-1 antibodies, the antibodies bind specifically to the target protein. (c) Detection staining: By using immunofluorescence technology, the protein bound to the antibody exhibits colour or fluorescence signals under a microscope. (d) Observation and image acquisition: Stained sections were observed under a microscope and images were obtained using the image processing software ImageJ. Quantitative measurements were conducted on the staining area using image analysis software to obtain values such as staining intensity and staining area.
Statistical analysis
The data in the study were entered using Excel spreadsheets and processed using statistical software SPSS 26.0. The econometric data that conformed to a normal distribution were described using (x ± s), and an F-value test was conducted, with a difference of p < 0.05 indicating statistical significance.
Results
Comparison of wound healing status
On the 1st day after wound formation, the wound healing area of three groups was consistent (p > 0.05). On the 7th and 14th days after wound formation, the wound healing area of three groups increased compared within groups, but only CG and OG wound healing areas were significantly higher than on the 1st day after wound formation (p < 0.05). Compared between groups, the wound healing area of CG and OG was higher than that of BG, while the wound healing area of OG was higher than that of CG (p < 0.05) – Table 1.
Table 1
Comparison of wound healing among three groups (x ± s) [cm]
After 1–14 days of wound formation, the wound healing area of all three groups increased. But compared to BG and CG, OG had a more significant increase in the wound healing area, followed by CG. The increase in BG was relatively small and there was no significant difference in Figure 1.
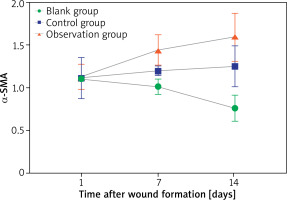
α-SMA comparison
On the 1st day after wound formation, the α-SMA of the three groups was consistent (p > 0.05). On the 7th and 14th days after wound formation, compared within groups, the α-SMA of BG decreased, while the α-SMA of CG and OG increased (p < 0.05). When comparing between groups, the α-SMA of CG and OG were higher than of BG, while the α-SMA of OG was higher than of CG (p < 0.05) – Table 2.
Table 2
Comparison of α-SMA levels among three groups (x ± s)
After 1–14 days of wound formation, the α-SMA of BG decreased, while the α-SMA of CG and OG increased. Compared to CG, the increase in α-SMA of OG was more significant – Figure 2.
COL-1 comparison
On the 1st day after wound formation, the COL-1 levels in the three groups were consistent (p > 0.05). On the 7th and 14th days after wound formation, compared within groups, the COL-1 of BG decreased, while the COL-1 of CG and OG increased (p < 0.05). When comparing between groups, the COL-1 of CG and OG was higher than that of BG, while the COL-1 of OG was higher than that of CG (p < 0.05) – Table 3.
Table 3
Comparison of COL-1 levels among three groups (x ± s)
After 1–14 days of wound formation, the COL-1 level of BG decreased, while the COL-1 levels of CG and OG increased. Compared to CG, OG had a more significant increase in COL-1 in Figure 3.
Discussion
Diabetes is a kind of chronic metabolic disease that is widely prevalent in the world. its main characteristics are high blood sugar level and insulin resistance, which may lead to damage to multiple organs and systems. Among them, DNHW is one of the common complications of diabetes patients. Because of the microcirculation disorder, nerve injury, immune function abnormalities and other factors caused by diabetes, the wound healing is seriously affected, and it is often difficult to recover [10]. The treatment of DNHW has certain challenges, and traditional treatment methods have limited effectiveness, so it is necessary to find new treatment strategies. In recent years, clinical attention has been increasingly focused on the role of immune microenvironment in wound healing. The immune microenvironment is a complex network formed by various immune cells, growth factors, and extracellular matrix factors around the wound, which plays an important role in regulating wound healing [11]. In patients with diabetes, the immune microenvironment may be subject to abnormal changes, resulting in difficult wound healing. As a traditional Chinese medicine, AST is considered to have multiple biological activities, including immune regulation, anti-inflammatory, antioxidant, and other effects. Based on this, major medical institutions have begun to pay attention to its potential effects in repairing DNHW.
This study preliminarily concluded through rat experiments that AST has a positive effect on accelerating the wound healing of DNHW. Studies have shown that [12] AST can promote the proliferation and differentiation of immune cells by regulating the activity and function of various immune cells, such as T lymphocyte (T cell), B lymphocyte (B cell), Macrophage (Mac), etc., thereby enhancing the immune response. And it can affect pro-inflammatory and anti-inflammatory cytokines, regulate the balance of cytokines, and adjust the degree and type of immune response. Based on this, the active ingredients such as FL, PS, and AA in AST have certain antioxidant effects. They can reduce the production of free radicals and oxidative damage, help protect wound tissue, promote cell proliferation and repair, and comprehensively improve wound healing speed [13, 14].
In addition, the expression of α-SMA in DNHW is closely related to wound repair and regeneration. The high expression of α-SMA usually implies the activation and proliferation of smooth muscle cells, which is an important step in the wound healing process. Increasing the expression of α-SMA can help promote wound repair, tightening of wound edges, and new angiogenesis, thereby improving the strength and stability of the wound [15, 16]. COL-1 is one of the main components of connective tissue such as skin, bones, and tendons. During the wound healing, the synthesis and accumulation of COL-1 help to provide structural support and mechanical strength for the wound, enabling it to better resist external pressure and tension. And the synthesis and arrangement of COL-1 can promote the contraction of collagen fibres at the edge of the wound towards the centre, which helps reduce the wound area. COL-1 can comprehensively accelerate the wound healing process by interacting with cell surface receptors, regulating cell signalling pathways, and affecting cell migration, proliferation, and differentiation [17, 18]. The active ingredients in AST can regulate the synthesis of extracellular matrix, which is a key component of wound structure support and cell migration. Their synthesis regulation directly affects the expression of α-SMA and COL-1. And FL and AA can affect the function of smooth muscle cells by affecting cell signal transduction pathways, while PS can promote new angiogenesis, provide more oxygen and nutrients. This affects the activity of smooth muscle cells and α-SMA in the wound, promotes the synthesis of collagen in the wound, and increases the levels of α-SMA and COL-1 [19, 20].
Conclusions
AST can regulate the immune microenvironment of DNHW, increase the levels of α-SMA and COL-1, and accelerate the wound healing of DNHW. However, this study still has some limitations and is prone to overlook the biological differences between rats and humans, including metabolic pathways and physiological mechanisms. Therefore, there may be some limitations in the conversion of rat experimental results to human disease treatment. And animal models may not fully simulate the full characteristics of human diseases, limiting the applicability of research. And rat experiments require longer time and higher costs, especially in long-term observation and multiple experiments, which further affects the accuracy and interpretability of the results. Therefore, further research and validation are still needed to translate experimental results into clinical applications.