Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic metabolic liver disorder [1] associated with obesity and metabolic disturbances [2]. It often results in necro-inflammatory responses and leads to various liver disorders [3]. While the underlying pathogenesis of NAFLD remains unclear, adverse metabolic profiles, such as dyslipidemia and insulin resistance, are known to be related to its development [4].
In modern medicine, pharmacological and nutritional interventions are considered the most important treatments for NAFLD and its related metabolic complications [5, 6]. However, accumulating evidence suggests that pharmacological treatment may have adverse effects on pruritus, lipid profiles and body weight [7]. Thus, nutritional interventions have been evaluated for their beneficial effects on NAFLD.
In this regard, vitamin D has emerged as a potential candidate for preventing the progression of NAFLD due to its association with insulin resistance, inflammatory action, and anti-fibrotic effects on the liver [8].
There has been considerable previous research on the relationship between vitamin D supplementation and NAFLD. However, the results have been conflicting. Some reports have demonstrated that vitamin D positively participates in glucose and lipid metabolism, inflammation, cellular differentiation, proliferation and apoptosis [9, 10]. Conversely, several trials have shown that supplemental vitamin D has an inverse effect on NAFLD [11, 12].
Several meta-analyses have been carried out to evaluate the effect of supplemental vitamin D on NAFLD, but the results have varied. For instance, a meta-analysis conducted by Sindhughosa et al. [13] found that additional vitamin D had a favorable influence on insulin resistance and reduced HOMA-IR in NAFLD patients. In contrast, another meta-analysis [14] showed no beneficial effect of additional vitamin D in the targeted NAFLD population.
Therefore, a systematic, up-to-date assessment incorporating additional trials is needed. The aim of our meta-analysis was to evaluate the potential predictive value of vitamin D supplementation on NAFLD.
Material and methods
Search strategy
We conducted systematic screening using PubMed, Embase, and the Cochrane Library for related publications in the last 5 years (from their inception to April, 2023), based on the relevant Medical Subject Heading (MeSH) terms and the following keywords: “vitamin D OR ergocalciferol OR cholecalciferol” AND “nonalcoholic fatty liver disease OR NAFLD OR nonalcoholic steatohepatitis” AND “efficacy”, in which we identified full-text papers from materials for further evaluation. Supplementary searches and hand-searching of the original retrieved studies and relevant articles were also performed to assess potentially eligible articles that were missed in the initial electronic search.
Eligibility criteria
Articles that were related to the following inclusion criteria were included in this analysis: 1) patients with NAFLD; 2) trials focused on comparing anthropometric and biochemical results, with vitamin D being the intervention, and placebo being the control; 3) more than one of the following parameters were mentioned in studies: high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), fasting blood glucose (FBG), homeostasis model assessment of insulin resistance (HOMA-IR), 25(OH)D and calcium; 4) the full texts of randomized control trials (RCTs) were included only.
The exclusion criteria were: 1) studies without a placebo or treatment group; 2) the data were incomplete, and unable to generate research outcomes; 3) the type of recruited article was non-RCT; 4) duplicated or overlapped previous literature.
Quality assessment
The literature screening process was performed separately by two reviewers. Quality of the literature was evaluated using the Newcastle-Ottawa Quality Assessment Scale. All the cohort articles were justified using the Cochrane Collaboration’s “Risk of bias” tool for the RCTs [15].
Data extraction
An independent data extraction form was used by two researchers to extract contents separately, including first author, publication year, country, interventions of vitamin D supplementation, number of patients, mean age, and outcomes of interest. Data were assessed. In case of disagreement, a third investigator would be involved to help resolve the disagreement.
Statistical analysis
The statistical analyses were conducted using Review Manager version 5.3 software. The heterogeneity across studies was examined by the I2 statistic [16]. An I2 value > 50% indicated a high degree of heterogeneity [17], where data were analyzed using a random-effect model. Otherwise, a fixed-effect model was used. The results were regarded as statistically significant with a p-value of < 0.05. We also applied mean difference (MD) and its 95% confidence interval (CI). The forest plots showed the aggregated results of our meta-analysis.
Results
Study selection process
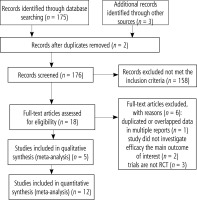
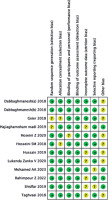
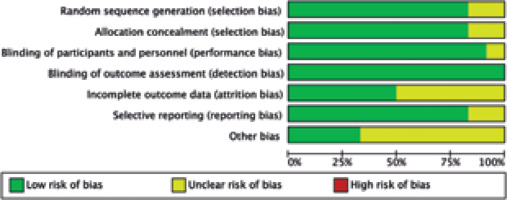
In total, 176 articles were identified initially from inception to April, 2023. Eighteen publications were evaluated by browsing the full studies in accordance with methods described in the literature, but some did not have sufficient data in the results. Finally, a total of 12 trials including 13 studies [9, 10, 18-27] were eligible for full-text assessment. In the trial conducted by Dabbaghmanesh, we included two studies with different interventions of vitamin D supplementation. The search process is shown in Figure 1. Moreover, Figures 2 and 3 summarize the quality assessment process. Table 1 describes clinical characteristics of 12 trials from 13 studies.
Table 1
Primary characteristics of the eligible studies
Synthesis of outcomes
Effect of vitamin D on lipid profile
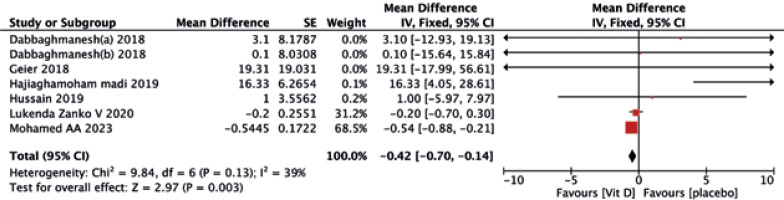
A statistically significant difference in serum LDL-C (MD = –0.42, 95% CI (–0.70, –0.14), p = 0.003) was observed following vitamin D intake compared to the placebo (Fig. 4).
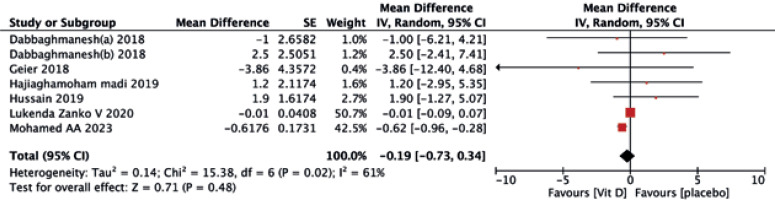
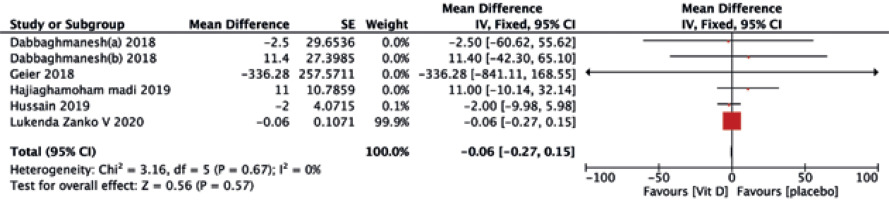
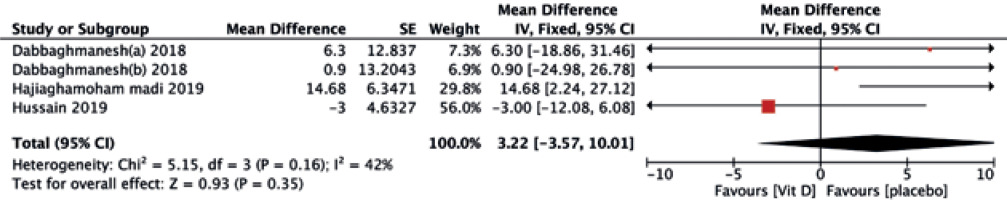
No significant differences were found between the two treatments in terms of HDL-C (MD = –0.19, 95% CI (–0.73, 0.34), p = 0.48) (Fig. 5), TG (MD = –0.06, 95% CI (–0.27, 0.15), p = 0.57) (Fig. 6), and TC (MD = 3.22, 95% CI (–3.57, 10.01), p = 0.35) (Fig. 7).
Effect of vitamin D on liver enzymes
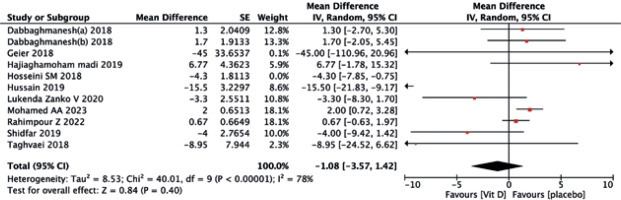
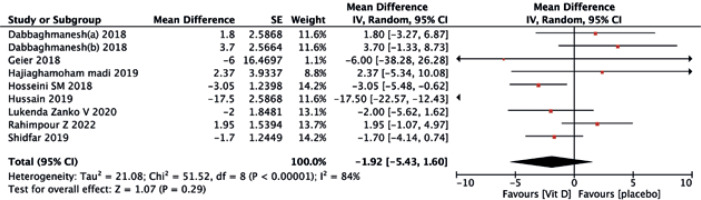
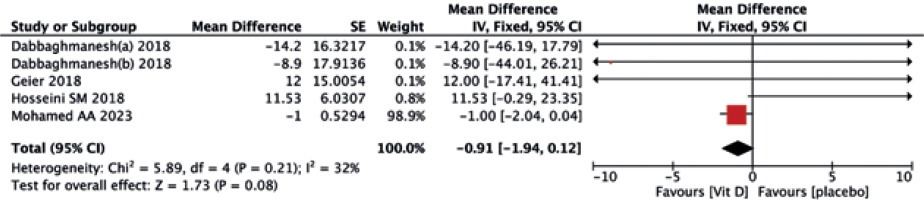
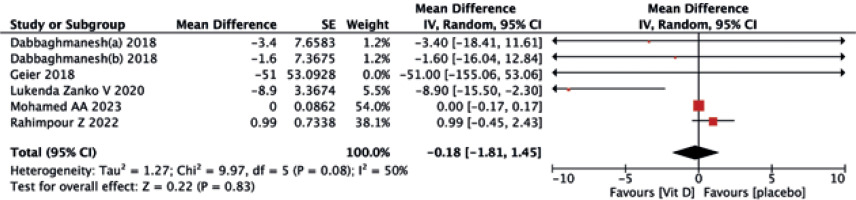
The vitamin D supplementation exhibited no significant impact on liver enzymes of ALT (MD = –1.08, 95% CI (–3.57, 1.42), p = 0.40) (Fig. 8) and AST (MD = –1.92, 95% CI (–5.43, 1.60), p = 0.40) (Fig. 9) when compared to the placebo group. Similarly, neither ALP (MD = –0.91, 95% CI (–1.94, 0.12), p = 0.08) (Fig. 10) nor GGT(MD = –0.18, 95% CI (–1.81, 1.45), p = 0.83) (Fig. 11) differed significantly between the two treatments.
Effect of vitamin D on glycemic indices
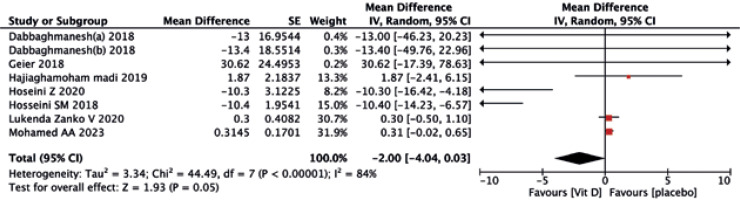
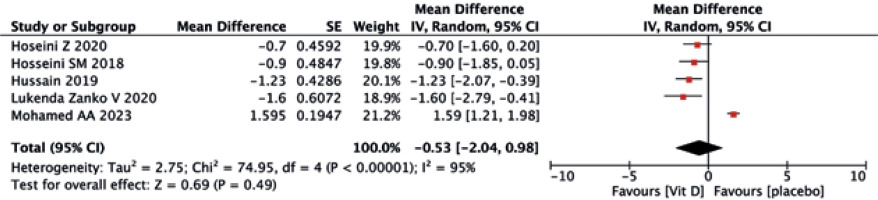
As shown in Figures 12 and 13, the vitamin D group did not have improved values of FBG (MD = –2.00, 95% CI (–4.04, 0.03), p = 0.05) and HOMA-IR (MD = –0.53, 95% CI (–2.04, 0.98), p = 0.49) compared to the controls.
Effect of vitamin D on calcium
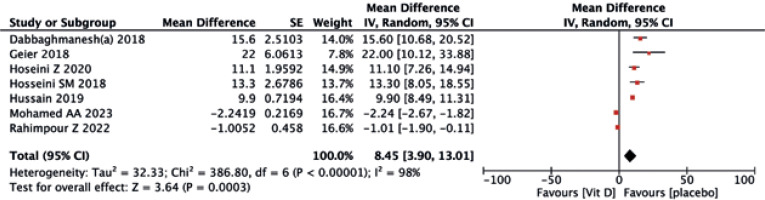
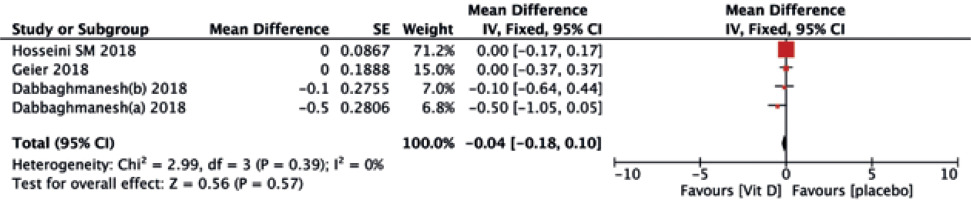
Seven trials reported an evident effect of supplemental vitamin D on serum 25(OH)D levels, with a statistically significant difference (MD = 8.45, 95% CI (–2.04, 0.98), p = 0.003) (Fig. 14). In terms of calcium, no differences were observed between the two treatments (MD = –0.04, 95% CI (–0.18, 0.10), p = 0.57) (Fig. 15).
Discussion
Non-alcoholic fatty liver disease is a metabolic disorder that has different clinical spectrums of liver disorders progressing from fatty liver to cirrhosis, and even hepatocellular carcinoma [28]. There is increasing scientific interest in the supplementation of vitamin D on NAFLD [29]. However, there continues to be debate about its effectiveness.
Several studies have examined the relationship between supplementing vitamin D and lipid profile in patients with NAFLD. A meta-analysis reported that vitamin D observably improved HDL-C without changing TG, TC and LDL-C in NAFLD [6]. However, Hussain et al. found that vitamin D did not exert a beneficial effect on lipid profile [22]. In another study, supplementing vitamin D decreased LDL-C, but no significant effect was found on HDL-C [19]. Our study also indicated that vitamin D supplementation significantly decreased LDL-C, without any effect on other lipid profile elements. The underlying mechanism for the impact of vitamin D on lipid profile is not fully understood, possibly due to the varying doses of vitamin D supplementation and duration of intervention in all included studies.
Our study identified a negative relationship between liver enzymes and vitamin D supplementation. The inconsistent findings from previous studies may be explained by other lifestyle and dietary elements that contribute to this result. Serum ALT levels have been reported to be associated with vitamin D intake and lifestyle changes [30]. Other research has revealed that a 12-week hypo-caloric diet combined with calcitriol increased ALT and AST in patients following vitamin D supplementation compared to the placebo group [11]. Additionally, it is logical to hypothesize that supplementing vitamin D may have no influence on the damaged liver due to chronic deficiency in vitamin D, but it may have preventative effects on NAFLD with dysmetabolic disorder and an increased risk of NAFLD.
Moreover, a systematic review by Guo et al. [31] reported the favorable influence of vitamin D addition on insulin resistance and insulin sensitivity. However, another systematic review [32] provided conflicting results, showing no association on insulin sensitivity with additional vitamin D, which was consistent with our meta-analysis. The underlying mechanism of vitamin D in affecting blood glucose is also largely unknown. It may be attributed to its potential role in regulating insulin secretion and levels of Ca2+. Calcitriol may elicit insulin secretion through a direct mechanism [24]. This is supported by the significant role of insulin-mediated intracellular pathways within muscle and adipose tissue [33, 34]. Furthermore, our findings suggested that supplementation of vitamin D had no association with alterations in calcium levels. Thus, further research is needed to address this issue.
There were a few limitations in our study. Firstly, it should be noted that due to the nature of the current retrospective meta-analysis, there is a potential for bias which may result in an imbalance between the groups being compared. Secondly, there may also be potential bias due to intrinsic differences in study design and inter-study heterogeneity. For example, different anthropometric and biochemical results may have an impact on the pooled outcomes. Therefore, further research is needed to identify subgroup participants who may benefit from vitamin D supplementation and refine the selection criteria accordingly.
Conclusions
To conclude, we demonstrated that additional treatment with vitamin D exerted an advantageous impact on 25(OH)D and LDL-C levels in patients with NAFLD. Nevertheless, it is currently unclear whether supplementing with vitamin D is beneficial for patients with NAFLD, based on the existing evidence. Hence, further research is warranted to investigate potential mechanisms underlying this correlation.