MicroNET-covered stent (CGuard) is a self-expandable 2nd-generation carotid dual-layer anti-embolic (“mesh”) stent with level-1 (randomized controlled trial) evidence for a profound reduction of peri-procedural cerebral embolism and elimination of lesion-related post-procedural embolism in carotid artery stenting (CAS) [1]. Clinical data demonstrate a minimized risk of 30-day death/stroke/myocardial infarction (≤ 1%) and optimal long-term outcomes with CGuard, in absence of device-related issues [2–6]. Today, competent CAS has a significant part in primary and secondary stroke prevention [7]. Also, evidence is increasing for an important role of the MicroNET-covered stent in improving the outcomes of emergency CAS in acute carotid-related strokes [8–10].
CGuard consists of a very widely open-cell (laser-cut) metallic frame (free cell area of ~22 mm²) that is wrapped by an outer, single-fiber knitted polyethylene terephthalate MicroNET adaptable sleeve (fiber thickness ~25 μm; cell size ~0.02–0.03 mm2; mesh fixation to the frame on stent edges [11, 12]); for a stent photograph see reference 13. CGuard combines properties of the most open-cell metallic stent frame (and thus very high conformability) with the smallest-cell anti-embolic layer [11, 12]. The MicroNET pore size is similar to that of embolic protection filters, resulting in a dense plaque coverage between the sparse struts, providing not only sequestration of the atherothrombotic plaque material but also a degree of sealing properties [13–17]. The MicroNET-covered stent shows no foreshortening or elongation and exhibits self-adaptability to the artery diameter (within the device nominal diameter; “SmartFit” characteristics) [11, 12]. The neuroprotective [1, 4, 14] stent has an increasing role in emergency management of carotid-related strokes [8–10]. Importantly, when properly implanted (post-dilatation embedded), the MicroNET-covered stent shows a normal healing profile and minimal in-stent restenosis (< 1%) [5, 6, 8, 17].
Embolic protection device use remains important in MicroNET-covered stent CAS because of the need to prevent cerebral embolism at procedural stages prior to protection by the MicroNET that is exerted only after the stent implantation and post-dilatation optimization [14]. Distal filters have several limitations relevant for cerebral safety of CAS [14, 18, 19]. Thus practical knowledge of how to effectively use proximal cerebral protection is crucial in today’s competent CAS [14, 20, 21]. For procedures at the level of carotid bifurcation, double-balloon systems, enabling transient endovascular exclusion of both the external (ECA) and common (CCA) carotid artery – and thus preventing any flow towards the brain in the internal carotid artery – are preferred [13, 14, 22] , as with a mono-balloon catheter the flow exclusion may be limited to CCA-only [13]. This, in some patients, can be insufficient for any effective cerebral protection because of the residual flow from the ECA to the ICA, towards the brain [13]. However, use of the double-balloon catheter [20] is not feasible in case of severe stenosis of the ECA ostium (Figures 1 A–C) and/or when the lesion involves distal CCA [13].
Figure 1
A 61-year-old man presenting with a recent right-hemispheric transient ischemic attack was treated, in primary prevention of carotid-related stroke, with proximal-protected stenting of the right internal carotid artery. MicroNET-covered 2nd-generation anti-embolic stent was used consistent with the PARADIGM protocol [11]. Proximal cerebral protection – offering a “protected” lesion crossing [14, 20] – was selected due to the lesion morphology (A, note a large ulceration in the right internal carotid artery extending to the distal common carotid artery seen with antegrade contrast injection, white arrows, via a non-inflated mono-balloon catheter, the catheter marker is indicated with a black arrow) and symptomatic presentation, consistent with the ‘tailored’ CAS algorithm [18]. With the external carotid artery (RECA) tight ostial stenosis, and lesion involving also the distal common carotid artery (A), use of a double-balloon proximal neuroprotection system (that is our preference [8, 10, 13]) was not feasible as the balloon-wire ECA exclusion system [22] was no longer available. Contrast injection (common carotid artery, CCA balloon inflated, B, white arrowheads, “back” pressure 68/52 mm Hg) demonstrated – with opening the stopcock of the system – flow reversal (B and C, dotted arrows) in the right internal carotid artery (RICA, target vessel), RECA, and CCA, consistent with an effective cerebral protection. The common carotid artery lesion was crossed with a coronary wire (BMW 0.014 J) and a 10 × 30 mm self-expandable MicroNET-covered stent was positioned (D, white arrowheads indicate the stent edges), released (E), and post-dilated with a 5.5 × 20 mm balloon (F). As post-aspiration visualization demonstrated stent presence in RECA-CCA (rather than ICA-CCA), an attempt was made to cross from the CCA to the ostial-stenosed (cf., A) – and now covered with the dual-layer stent – RICA. Attempts to cross with standard coronary wires (BMW 0.014” and WhisperMS 0.014”) failed but crossing with a V-14 wire (with manually modified tip angulation to resolve the “wire-preferred” entry into the RICA ulcer, yellow arrows in G) was successful (H, green arrow). Effective reaching of the RICA distal extracranial segment with the angioplasty wire is shown in I. Insertion of a small coronary balloon through the 2 stent layers and the RICA ostial stenosis required increased support from the guiding catheter (note the catheter marker within the proximal portion of the 1st stent, J, arrow). Gradual opening of the RICA ostium was performed under resumed (due to extended procedure duration – at key steps) proximal protection, using a semi-compliant coronary balloon (3.5 × 15 mm, K) followed by non-compliant balloons (5.0 × 20 mm and 6.0 × 20 mm, L and M). With the above preparation, a second 10 × 30 mm self-expandable MicroNET-covered stent could be smoothly inserted into the RICA, positioned (N), and released (O). The stent was gradually post-dilatation optimized, up to using (finally) an 8.0 × 20 mm balloon inflated up to 20 atm at the proximal segment (P). No balloon inflations from the CCA to RECA were performed following the 2nd (ie., RICA-RCCA) stent implantation. The final result at the carotid bifurcation is shown in Q (non-subtracted image) and R (digital subtraction angiogram). There was no cerebral embolism and the procedure was clinically uneventful. Annual clinical and duplex ultrasound follow-ups were normal. At 5 years in-stent peak velocities remained normal (RICA – 58/28 cm/s; RECA – 82/21 cm/s; RCCA – 76/31 cm/s), and computed tomography angiography demonstrated – with the “Y” configuration of the stents – a lasting optimal anatomic result of the reconstruction of carotid bifurcation in absence of any restenosis (S, T).
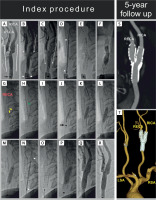
We present procedural imaging demonstrating how to resolve safely and effectively – using the endovascular route – an accidental implantation of the CGuard double-layered stent into the ECA (rather than ICA), covering the (diseased) ICA ostium with the MicroNET and strut structure; this occurred in CAS employing a mono-balloon catheter with transient flow reversal for cerebral protection (Figures 1 A–R). 5-year follow-up showed a maintained excellent anatomic result (Figures 1 S-T) in the context of uneventful clinical follow-up.
In conclusion, with mono-balloon use for proximal protection in CAS/eCAS, landmark separation of the ICA and ECA is critical to avoid accidental stent placement in ECA. We show that inadvertent placement of the dual-layer MicroNET-covered stent can be resolved, using the endovascular route (same, continued procedure), by (1) crossing the MicroNET and stent strut frame and making a step-wise gradual opening onto the ICA, followed by (2) placement and optimization of another MicroNET-covered stent (appropriately positioned in the ICA; “Y” technique). This endovascular resolution was safe and effective, with an optimal clinical and anatomic result at long-term. Today, ensured separation of ICA from the ECA under mono-balloon catheter proximal cerebral protection can be practiced – along with neurovascular interventions – in a novel human stroke model [23].








