Introduction
The annual number of surgical procedures conducted globally is approaching 200 million. Major gastrointestinal cancer surgery is illustrative of this population because it affects millions of people every year [1]. One of the most difficult aspects of providing medical treatment on a worldwide scale is dealing with patients who require high-risk procedures that do not include heart surgery. Additionally, within a month of surgery, almost 10 million people encounter significant perioperative cardiovascular problems [2]. The reason for this is that people who have undergone elective major gastrointestinal surgery with a high risk of postoperative morbidity (including cardiac ischaemia episodes) may not be able to complete tests like metabolic equivalency tests that objectively assess the cardiorespiratory reserve [3]. Perioperative cardiac events (including cardiac arrest, heart failure, myocardial infarction, and arrhythmias) account for between 1% and 7% of all deaths and hospitalisations. The frequency of these incidents has remained relatively constant despite decades of study into their predictability and prevention [4].
Epidural analgesia has been studied for its potential advantages after surgery in several randomised clinical trials, with most finding improvements in pain and secondary endpoints like the incidence of postoperative complications. Although the danger of haematoma can be mitigated with epidurals [5], other benefits besides pain alleviation have been explored to strike a better balance [6]. Despite a clinically significant trend, neither meta-analysis had enough participants to reveal a statistically significant difference in death. The use of epidural analgesia is still debatable in this setting because the possibility of a significant decrease in mortality and serious complications from using epidural analgesia in cardiac surgery is balanced by the prospect of a greater haematoma risk than in non-cardiac surgery.
Inadequate sample sizes in mortality estimates and the persistent worry that the risk of epidural haematoma may be enhanced in heart surgery both represent gaps in the current literature. In case reports, the incidence is overstated because the denominator (the number of epidurals administered to develop the haematoma) is not recorded. However, if only randomised trials are studied, the incidence of haematoma is insignificant and equal to zero.
Aim
The current analysis aims to measure the relationship between epidural analgesia and cardiac outcomes expressed by myocardial infarction, and also to measure the influence of epidural analgesia on the mortality rate compared with a control group.
Material and methods
Study design
The epidemiological declaration includes meta-analyses of recent clinical research that followed a predetermined study strategy. Several scientific databases, including OVID, Cochrane Library, PubMed, Embase database, and Google Scholar, were used for data gathering and analysis of recruited studies according to the inclusion criteria.
Data pooling
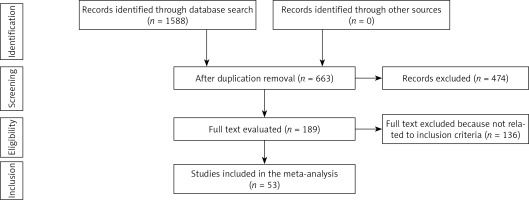
Analysing the effects of various outcomes was done using retrospective studies that looked at the influences of epidural and general analgesic anaesthesia techniques on mortality rates and postoperative cardiac outcomes. All studies were human-related, regardless of language. The sample size of studies that were recruited did not have any limitations. Communications, editorials, reviews, and letters were not included in the current meta-analysis because they are non-interventional research. The process of study selection and inclusion is shown in Figure 1.
Eligibility and inclusion
Examining the effect of various analgesic techniques on postoperative outcomes in surgical subjects, a summary was generated.
The sensitivity study only included articles that discussed how interventions affected the frequency of post-surgical myocardial infarctions and mortality rates. For subclass and sensitivity analysis, various subject types were compared to the interventional groups.
Inclusion criteria
The acceptable studies to be included in the current analysis should be randomised clinical trials published up to April 2023.
Patients undergoing surgery requiring analgesia made up the target intervention population.
The included studies’ intervention plans were compared the postoperative results of generalised analgesia with epidural analgesia.
Identification
A protocol of search strategies was defined in accordance with the PICOS principle as follows: P (population) surgical patients Anaesthesia is the I (intervention/exposure); various anaesthesia interventions are the C (comparison). O (outcome): Mortality and postoperative myocardial infarction; R: randomised clinical studies. S: study design.
The authors performed a thorough search of the Cochrane Library, PubMed, Embase, OVID, and Google Scholar databases up to April 2023 using the keywords and related terms given in Table I. Any article that did not discuss and evaluate the various analgesia during surgery procedures and perioperative cardiovascular outcomes was disregarded after an evaluation of the article titles and abstracts, which had been collected into a reference managing program. Q.H. and T.Z., the 2 authors, served as reviewers to find pertinent studies.
Table I
Search strategy for each database
Screening
The data were narrowed down in accordance with the following criteria: the surname of the first author, the year of publication, the country in which the study was conducted, the design of the study, the population type recruited in the studies, the duration of the study, demographic information, clinical and treatment characteristics, the total number of subjects, study-related features presented in a standard format, the information source, and the outcome. Each study was examined to determine whether it had any kind of bias, and then the methodological quality of the selected papers was analysed in a blind fashion by 2 different writers.
The potential for bias in each of the included studies was evaluated with the help of the software package Review Manager, and the results were classified into one of 3 categories: low, intermediate, or high potential for bias. Each study was evaluated methodologically by 2 separate reviewers.
Statistical analysis
In the present meta-analysis, a random-effect model was used to obtain the mean difference (MD) along with a confidence interval (CI) that ranged from 0 to 95%. A random-effects model was fitted to the data. Using the constrained maximum-likelihood estimator, the level of heterogeneity (τ2) was calculated. The I2 index, which is a numerical number with a range from 0 to 100 and is conveyed in the form of Forrest plots, was computed. This index was obtained using the software package Review Manager. The heterogeneity level was shown by percentages ranging from 0% to 100%, and it was also expressed by percentages indicating low, moderate, and high levels of heterogeneity. Begg’s and Egger tests were used to conduct quantitative research on publication bias, and the presence of publication bias was deemed to be present if p > 0.05. A test with 2 possible outcomes was performed to derive the p-values. Using the dichotomous model, the statistical analyses and graphs were displayed with the software Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and Jamovi software version 2.3.
Results
After reviewing 1588 pertinent studies, 53 from the period of 1987 to 2019 were included in the meta-analysis because they met the inclusion criteria [7–59]. The results of these investigations are compiled in Table II (characteristics of included research including year, country, subject count, and study quality).
Table II
Characteristics of included studies
| Study | Year | Country | Epidural group (n) | Control group (n) | Total number of subjects | Risk of bias |
|---|---|---|---|---|---|---|
| El-Baz [47] | 1987 | USA | 30 | 30 | 60 | High |
| Rein [22] | 1989 | Norway | 8 | 8 | 16 | Low |
| Liem [33] | 1992 | Netherlands | 27 | 27 | 54 | High |
| Kirnö [35] | 1994 | Sweden | 10 | 10 | 20 | Low |
| Stenseth [17] | 1994 | Norway | 20 | 10 | 30 | Low |
| Moore [28] | 1995 | UK | 9 | 9 | 18 | Intermediate |
| Levang [16] | 1996 | Norway | 27 | 27 | 54 | Low |
| von der Linden [14] | 1996 | Sweden | 14 | 13 | 27 | Intermediate |
| Fawcett [46] | 1997 | UK | 8 | 8 | 16 | Intermediate |
| Brix-Christensen [54] | 1998 | Denmark | 8 | 8 | 16 | High |
| Chae [51] | 1998 | Korea | 12 | 12 | 24 | Intermediate |
| Mehta [30] | 1998 | India | 25 | 25 | 50 | Intermediate |
| Loick [32] | 1999 | Germany | 25 | 47 | 72 | High |
| Tenling [15] | 2000 | Sweden | 15 | 15 | 30 | Intermediate |
| Dhole [48] | 2001 | India | 21 | 20 | 41 | Intermediate |
| Jideus [39] | 2001 | Sweden | 45 | 96 | 141 | Intermediate |
| Scott [12] | 2001 | UK | 206 | 202 | 408 | Low |
| Bach [58] | 2002 | Germany | 13 | 27 | 40 | Intermediate |
| De Vries [49] | 2002 | Netherlands | 30 | 60 | 90 | High |
| Fillinger [45] | 2002 | USA | 30 | 30 | 60 | Low |
| Priestley [10] | 2002 | Australia | 50 | 50 | 100 | Intermediate |
| Berendes [55] | 2003 | USA | 36 | 37 | 73 | Intermediate |
| Volk [11] | 2003 | Germany | 13 | 13 | 26 | Intermediate |
| Kendall [38] | 2004 | Ireland | 10 | 20 | 30 | Low |
| Barrington [56] | 2005 | Australia | 60 | 60 | 120 | High |
| Kessler [37] | 2005 | Germany | 30 | 30 | 60 | Intermediate |
| Kiliçkan [36] | 2005 | Turkey | 40 | 40 | 80 | Intermediate |
| Lundstrøm [31] | 2005 | Denmark | 30 | 25 | 55 | Intermediate |
| Hansdottir [43] | 2006 | Sweden | 58 | 55 | 113 | Intermediate |
| Bakhtiary [57] | 2007 | Germany | 66 | 66 | 132 | Intermediate |
| Hejimans [42] | 2007 | Netherlands | 15 | 45 | 60 | Intermediate |
| Jakobsen [41] | 2007 | Denmark | 10 | 10 | 20 | High |
| Royse [21] | 2007 | Australia | 37 | 39 | 76 | Intermediate |
| Salvi [20] | 2007 | Italy | 389 | 389 | 778 | Low |
| Caputo [53] | 2009 | UK | 36 | 38 | 74 | Low |
| Rodriguez [24] | 2008 | Spain | 10 | 12 | 22 | Intermediate |
| Crescenzi [50] | 2009 | Italy | 46 | 46 | 92 | High |
| Mehta [29] | 2010 | India | 31 | 31 | 62 | Low |
| Sharma [19] | 2010 | India | 30 | 30 | 60 | Intermediate |
| Caputo [52] | 2011 | UK | 109 | 117 | 226 | Intermediate |
| Onan IS [25] | 2011 | Turkey | 15 | 15 | 30 | High |
| Porizka [23] | 2011 | Czech Republic | 15 | 15 | 30 | Low |
| Svircevic [7] | 2011 | Netherlands | 327 | 329 | 656 | Intermediate |
| Amat-Santos [59] | 2012 | Canada | 74 | 61 | 135 | High |
| Jakobsen [40] | 2012 | Denmark | 31 | 31 | 62 | Intermediate |
| Liang [34] | 2012 | China | 32 | 32 | 64 | Intermediate |
| Gurses [44] | 2013 | Turkey | 32 | 32 | 64 | Intermediate |
| Nešković [27] | 2013 | Serbia | 35 | 46 | 81 | Low |
| Onan B [26] | 2013 | Turkey | 20 | 20 | 40 | Intermediate |
| Stenger [18] | 2013 | Denmark | 508 | 508 | 1016 | High |
| Toda [13] | 2013 | Japan | 7 | 7 | 14 | Low |
| Mohamad [9] | 2017 | Egypt | 60 | 60 | 120 | Low |
| Elzohry [8] | 2019 | Egypt | 30 | 30 | 60 | Low |
Myocardial infarction
All the included articles were judged for the postoperative myocardial infarction, with total of 5898 surgery subjects. The analysed data showed a significant drop in myocardial events in the epidural analgesia group compared with the control group (MD = 0.66, 95% CI [0.49, 0.88], p = 0.005) with no heterogeneity (I2 = 0%) (Figure 2). In accordance with subgroup analysis, the findings of analysis of the low- and high-risk groups of the included studies showed a significant impact of epidural analgesia compared with traditional analgesia on postoperative myocardial infarction (MD = 0.50, 95% CI [0.31, 0.83], p = 0.03 and MD = 0.56, 95% CI [0.34, 0.94], p = 0.007, respectively) (Figure 3). In contrast, analysis of studies that classified as intermediate risk of bias showed non-significant difference between the intervention and control groups (MD = 0.91, 95% CI [0.57, 1.47], p = 0.7) (Figure 3).
Figure 2
Forest plot indicating the impact of epidural anaesthesia versus control on incidence of myocardial infarction

Figure 3
Forest plot indicating the impact of epidural anaesthesia versus control for high (A), moderate (B), low (C) risk of bias studies on incidence of myocardial infarction

Heterogeneity analysis for different models for analysis of myocardial infarction showed I2 = 0 for 3 models (overall [Figure 2], high-risk group [Figure 3 A], and moderate-risk group [Figure 3 B]), while the low-risk group showed a low heterogeneity expressed as I2 = 39%, as shown in Figure 3 C.
Mortality
There were 37 studies included in the meta-analysis, with a total of 4910 individuals, all of whom had received both epidural analgesia and general anaesthesia throughout their surgeries. Epidural analgesia was associated with a significantly lower number of death cases in the interventional group compared to the control group (MD = 0.59, 95% CI [0.41, 0.85], p = 0.005) (Figure 4). However, subgroup analysis revealed no statistically significant differences between the 2 sets of participants. Figure 5 displays the results of a study of high, intermediate, and low risk of bias subgroups: (MD = 0.62, 95% CI [0.30, 1.28], p = 0.2), (MD = 0.81, 95% CI [0.43, 1.53], p = 0.51), and (MD = 0.49, 95% CI [0.09, 2.71], p = 0.41).
Figure 5
Forest plot indicating the impact of epidural anaesthesia versus control for high (A), moderate (B), low (C) risk of bias studies on mortality rate

Heterogeneity analysis for different models for analysis of mortality rate showed I2 = 0 for 3 models (overall [Figure 4], moderate-risk group [Figure 4 B], and low-risk group [Figure 4 C]), while the high-risk group showed a low heterogeneity expressed as I2 = 41%, as shown in Figure 4 A.
Additionally, Begg’s and Egger tests were used to evaluate publication related bias, which revealed a non-significant bias for all included analysis groups with a p-value greater than 0.05. For the MI analysis of all studies, the Begg’s test p-values were 0.94 and the Egger test result was 0.45.
As stated in Table II, the risk of bias assessment was assessed. For analysis related to postoperative myocardial infarction, 11 studies showed high risk, 15 studies showed low risk, and 27 studies showed intermediate risk of bias. While for mortality-related analysis there were 10 studies with high risk, 4 low-risk studies, and 23 studies with intermediate risk.
Discussion
Fifty-three studies in total were gathered for the current analysis to examine the effects of various anaesthesia procedures (epidural and generalised) on the outcomes following surgery.
When compared to conventional analgesia, it was discovered that epidural analgesia produced more favourable cardiac outcomes. There was a statistically significant difference between the number of myocardial infarctions in the epidural analgesia group and the control group at all 3 levels of statistical significance (p = 0.005, p = 0,007, and p = 0.03 for total included studies, high risk of bias, and low risk of bias, respectively). This difference was seen at all 3 levels of statistical significance. Analyses with a moderate risk of bias did not uncover any significant differences between the groups (p = 0.7). Epidural analgesia did not significantly reduce postoperative mortality in studies with high risk, middle risk, or low risk (p = 0.13, p = 0.51, and p = 0.41, respectively).
Even though some of the publications that were included in this meta-analysis may have used somewhat different definitions, the majority of the authors correctly characterised myocardial infarction as the presence of both increased cardiac biomarkers and electrocardiographic abnormalities. This is the case even though some of the authors may have used slightly different definitions. It is well-established in the field of cardiac surgery that a rise in cardiac biomarkers following CABG surgery indicates myocyte necrosis. This indicates that a larger biomarker magnitude is likely to be associated with a worse prognosis.
Open surgical procedures and laparoscopy induce inflammation, hypercoagulability, and discomfort, hence elevating the likelihood of myocardial ischaemia. The occurrence of myocardial ischaemia and infarction during laparoscopic surgery is rather rare compared with open surgery [60]. The advent of laparoscopic surgery has revolutionised post-operative care and significantly decreased the duration of hospitalisation, allowing many surgical operations to be performed on an outpatient basis. The likely cause of this is the low rate of physiological disruptions and stress associated with laparoscopy. Early discharge is advantageous for patients and should be standard practice once they no longer require in-hospital care [61]. A-VATS allows the surgeon to access the tissue through a small incision, resulting in little lung exposure to air pressure. This technique has the advantage of causing less postoperative respiratory dysfunction compared to open surgery. Additionally, even when a bilateral approach is used in a single session, the morbidity rate is reduced [62]. Due to recent advancements in laparoscopic surgery, this method is now the favoured choice in over 50% of thoracic surgery patients. Therefore, it is utilised to a greater extent and with broader application, particularly at specialised and proficient thoracic surgical centres [63].
Thoracic epidural analgesia, with or without premedication, is a commonly employed technique in video-assisted thoracic surgeries (VATs). The anaesthetist positions the thoracic epidural needle between the T4 and T6 vertebrae, resulting in a somatosensory and motor block within the T1-T9 region. The continuous administration of local anaesthesia infusion can be used to sustain this effect [64].
Since Yeager et al. [65] determined that postoperative epidural analgesia decreased the overall complication rate in high-risk patients, the efficacy of epidural analgesia in preventing postoperative cardiac morbidity has been the subject of much debate. The study’s design (it was not blinded, and the control group did not receive very good analgesia), the decision to end the study early (because of the huge disparity in outcomes between the 2 groups of patients) and the overall high rate of morbidity sparked heated debate after the article was published. We analysed how not having this article in the collection would affect things. At this level of mortality, the remaining trials lack sufficient power to draw any conclusions. Standardised procedures for conducting a meta-analysis are essential. A priori question formulation, a well-described search strategy, study selection, and reliable data analysis are all essential steps. The methods developed for this study have been used by researchers investigating interventions for better cardiac outcomes in surgery subjects. We were careful not to include multiple papers on the same patients, which could introduce bias. In our opinion, this research meets all the requirements for a sound meta-analysis. As our major method of analysis, we settled on the random effects model. To determine the impact of alternative modelling, we utilised sensitivity analysis. The net result is within 5%, while the fixed effects model yields similar results.
Even though the majority of these randomised clinical trials involved low-risk patients undergoing coronary artery bypass surgery [66], previous meta-analyses have demonstrated a significant benefit with epidurals for a combined outcome of mortality and myocardial infarction, ventilation time, pulmonary complications, and supraventricular tachyarrhythmias [67]. Neurological impairment, including paraplegia, can develop from an epidural haemorrhage during any clinical condition, including non-cardiac surgery, pain management, and childbirth.
Epidural anaesthesia for heart surgery carries a known risk of catheter-related haemorrhage. The risk of epidural haemorrhage from the complete anticoagulation necessary for CPB has been the subject of some discussion in cardiac surgery [6, 68]. However, patients with epidural haematoma were not documented until 2004 [69], and the sole estimate made prior to that was based on mathematical modelling of unproven events, which led to extremely large confidence intervals of risk, ranging from 1–1500 to 1–150,000 patients [70]. An estimated incidence of haematoma in cardiac surgery in 2007 was the most recent risk assessment, which was reported in 2008 by Wijeysundera et al. [71]. A risk of decompression laminectomy of 1 : 7246 (95% CI: 1–5000 to 1–10) was also observed by the authors [5]. Using a sample size nearly double that reported by Wijeysundera et al., we discovered that the use of high thoracic epidural analgesia in cardiac surgery, where full anticoagulation is not necessary, is not associated with an increased risk of epidural haematoma [71]. It is possible that epidural haematomas are caused by excessive blood loss during catheter insertion or removal, as well as by repeated punctures, bloody taps, poor anticoagulation, or excessive antiplatelet medication. During the preoperative period, anaesthesia should involve standard assessments of patients’ cardiovascular and pulmonary risk status. Additionally, electrocardiography (ECG), peripheral oxygen saturation, blood pressure, and end-tidal carbon dioxide levels should be thoroughly evaluated and explained. Special emphasis is necessary to ensure surgical success and the safety of the patient in cases of awake VATS anaesthesia [64, 72].
Regarding other cardiac outcomes post-surgery, according to Gurses et al. [44], it was clear that significantly more people in the control group developed postoperative hypertension (p = 0.001). Postoperative dysrhythmia, bradycardia, hypotension, and the requirement for inotropic medications occurred with similar frequency in both groups.
After cardiac surgery, patients who received epidural analgesia for longer than 24 h experienced less myocardial infarction, according to the current meta-analysis which is consistent with previous meta-analysis conducted by Beattie et al. [73]; these findings were similar to other studies [66, 67], while the previous meta-analysis did not provide evidence that TEA has a different effect on short- or long-term mortality when considered separately, which raises questions about the validity of these findings.
Many studies that could have produced a different outcome were not included in the meta-analysis. However, because these studies did not meet the requirements for inclusion in our meta-analysis, we did not include them. Additionally, not all the included studies evaluated how race may have an impact on the demonstrated outcomes, so we could not determine whether the observed patterns are racially motivated. According to the NOS score, the methodological quality of some of the research we considered is quite low. Unpublished literature and uncollected data can introduce bias into a study.
Conclusions
Epidural analgesia has a significant impact and protective cardiac effect through reduction of postoperative myocardial infarction events among surgery subjects, while the impact on mortality was similar to the traditional generalised anaesthesia. However, future clinical multicentre studies are needed to draw more solid conclusions.