Summary
Our study demonstrated that lower IL-33 and higher ST2 serum concentrations, as well as older donor age, larger left ventricular diastolic dimension and longer time from heart transplantation to blood collection, are independently associated with cardiac allograft vasculopathy (CAV). Among the independent factors, IL-33 and ST2 have the strongest predictive power for the detection of CAV. The combined assessment of IL-33/ST2 in one model significantly increases the predictive power, sensitivity and specificity for the identification of patients with CAV. This study may have clinical implications because it provides noninvasive, low-cost, and simple indicators for CAV detection.
Introduction
Cardiac allograft vasculopathy (CAV) is a diffuse concentric hyperplasia of the epicardial coronary arteries as well as intramyocardial small arteries and arterioles that constitute the microcirculation of the transplanted heart [1–3]. Furthermore, CAV can also affect cardiac veins [1, 2]. Numerous alloantigen-dependent or alloantigen-independent factors contribute to the development of CAV, including human leukocyte antigens (HLA) mismatch, the presence of alloreactive antibodies, cytomegalovirus (CMV) infection, donor age, donor cause of death, ischemic time, episodes of acute rejection and classic cardiovascular risk factors such as diabetes mellitus, hypertension and hyperlipidemia, which can be enhanced by immunosuppressive therapy [3–7]. Immunological events are considered to be the principal initiating stimuli, causing endothelial damage and altered endothelial permeability [3, 4].
CAV is mainly a disease of the intima, and early lesions include intimal thickening, mild fibrosis and increases in extracellular matrix proteins. Lesions then progress to diffuse fibrous intimal hyperplasia, appear along the entire length of the affected vessel and lead to luminal stenosis and occlusion in its later stages. On the basis of endomyocardial biopsies, it has been postulated that endothelial damage develops at first in the microcirculation [3, 4]. Furthermore, due to allograft denervation, CAV often develops asymptomatically until heart failure, cardiac arrhythmia or sudden death occurs.
Thus, the early detection and diagnosis of CAV using coronary angiography or intravascular ultrasound are difficult [1–8]. Therefore, the identification of simple biomarkers in the blood associated with the presence of CAV would be beneficial in identifying potential associations that could be targeted with new therapies. Among the relatively new biomarkers that have a potential role in the development and progression of CAV are interleukin-33 (IL-33) and suppression of tumorigenicity 2 (ST2).
The IL-33/ST2 pathway plays an important role in inflammation as well as in modulating both innate and adaptive immune responses [9–11]. Furthermore, this pathway contributes to the maintenance of tissue homeostasis [9–11]. IL-33 can induce the switch from T helper 1 (Th1) to T helper 2 (Th2) cell differentiation [10, 11]. Th1 activation evokes cell-mediated immunity and phagocyte-dependent inflammation, and Th2 cell activation is associated with antibody responses and inhibits several functions of phagocytic cells. Episodes of acute and chronic rejection following heart transplantation (HT) are believed to result from a Th1 cell-dominated immune response. In addition, Th1 activation underlies inflammatory disorders, including atherosclerosis. In contrast, the Th2-type response has been implicated in graft tolerance during allograft rejection [12, 13]. IL-33/ST2 signaling also regulates protein expression in endothelial cells and can also be involved in the development of vascular endothelial dysfunction [14]. Furthermore, the IL-33/ST2 pathway influences local fibrosis in coronary vessels by influencing inflammatory cell infiltration [10–12].
Considering the influence of the IL-33/ST2 pathway on endothelial dysfunction, possible proinflammatory and fibrotic effects and modulation of the immune response, we speculated that these markers could be potentially associated with the development and progression of CAV.
Aim
This study aimed to determine the factors associated with CAV detection, with particular emphasis placed on the role of IL-33 and ST2.
Material and methods
We investigated 347 consecutive heart transplant recipients who attended as part of their routine, posttransplant annual review during the period 2016–2018. Patients with malignancy (n = 8), inflammatory musculoskeletal disorders (n = 6), connective tissue diseases (n = 4), and infectious diseases (n = 8) at the time of enrollment were excluded from the study. To distinguish CAV from passenger atherosclerosis we excluded from the analysis patients with lesions in coronary arteries in the first CAG at 1 year after HT (n = 21). The resulting study sample comprised 299 patients.
During the visit to each patient, a panel of laboratory tests, determination of the level of immunosuppressive drugs, measurement of IL-33 and ST2 serum concentrations, echocardiography and coronary angiography (CAG) were performed. Data on basic characteristics and medical treatment were collected by interviewing the patient and reviewing the electronic records. In addition, data relating to donor details, such as age, sex and ischemic time, were collected. The CAG was used to determine vasculopathy status and was compared with previously available CAGs. All CAGs were reviewed by two independent, experienced, interventional cardiologists to accurately classify coronary artery lesions [15]. In case of divergent results, the opinion of a third experienced angiographer was taken into account. The group without CAV was defined as having no coronary vessel lesions, while the CAV group comprised patients from CAV 1 to CAV 3. CAV was diagnosed in case of evidence of narrowing or luminal irregularities either in the left main or any primary or branched coronary vessels, as observed on the CAGs. Patients with prior lesions requiring percutaneous coronary intervention were classified as having severe vasculopathy, even if no severe lesions were seen.
CAGs were performed routinely in all patients 1 year after HT and then repeated every 2 years if no lesions in coronary vessels were found or every year if the lesions were present. All patients received intracoronary nitroglycerin before intravenous contrast injection. For each patient, we recorded the time from HT until the first CAG showing any degree of CAV.
Acute cellular rejection episodes were confirmed on the basis of specimens obtained from the endomyocardial biopsies (EMBs) performed per center protocol starting 1 week after HT and were repeated every week during the first month after HT followed by the EMBs obtained at the end of the 6th and 8th week, and the 3rd, 6th, 9th and 12th month after HT.
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Medical University of Silesia in Katowice (protocol code: KNW/0022/KB1/142/16 and date of approval: 29/11/2016). Informed consent was obtained from all subjects involved in the study.
Treatment
Treatment after HT was carried out according to the protocol of our center. No induction therapy was used in our study group. Maintenance therapy was performed with tacrolimus or cyclosporine in combination with mycophenolate mofetil with steroid withdrawal 12 months after HT. Serum tacrolimus levels were kept between 8 and 12 μg/ml for the first 3 years and 5–8 μg/ml after that time. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors were used independently of cholesterol levels in all patients tolerating this therapy. All patients except CMV-seronegative recipients of a CMV-seronegative donor received CMV prophylaxis with ganciclovir or valganciclovir in the first 3 months after HT.
Biochemical measurements
Samples of peripheral venous blood for routine laboratory parameters were drawn after 12 h of fasting from the antecubital vein on admission and studied at the laboratory within 30 min of collection. The complete blood count and hematologic parameters of patients were analyzed using automated blood cell counters (Sysmex XS1000i and XE2100, Sysmex Corporation, Kobe, Japan). The intra- and interassay coefficients of variation of the blood samples were 5% and 4.5%, respectively. Hepatic and renal function parameters, as well as cholesterol and albumin plasma concentrations, were determined with a COBAS Integra 800 analyzer (Roche Instrument Center AG, Rotkreuz, Switzerland). The plasma concentration of fibrinogen was measured using an STA Compact analyzer (Roche). Erythrocyte sedimentation rate was measured using the Westergren method.
Fasting venous samples for human IL-33 and human ST2 measurements were drawn from the antecubital vein and frozen at –80°C. Human IL-33 was measured by sandwich enzyme-linked immunosorbent assay (ELISA) with a commercially available kit (Human IL-33 ELISA, SunRedBio Technology Co, Ltd, Shanghai, China). The concentration of IL-33 was expressed as ng/l. The sensitivity of the assay was 0.573 ng/l. Assay range was 0.6–180 ng/l. This ELISA test was performed using a BioTek Elx50 reader (BioTek Instruments Inc., Tecan Group, Switzerland). Human ST2 was measured by sandwich enzyme-linked immunosorbent assay (ELISA) with a commercially available kit (Human ST-2 ELISA, SunRedBio Technology Co, Ltd, Shanghai, China). The concentration of ST2 was expressed as ng/ml. The sensitivity of the assay was 0.436 ng/ml. Assay range was 0.5–150 ng/ml. This ELISA test was performed using a BioTek Elx50 reader (BioTek Instruments Inc., Tecan Group, Switzerland).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Medical University of Silesia in Katowice (protocol code: KNW/0022/KB1/142/16 and date of approval: 29/11/2016).
Statistical analysis
The statistical analysis was performed using SAS software (version 9.4). Continuous variables were expressed as the mean (standard deviation) or median with upper and lower quartiles according to variable distribution. Differences in continuous variables were compared using the independent Student’s t-test for normally distributed variables and the Mann-Whitney U test for nonnormally distributed variables. Categorical variables were expressed as numbers and percentages and compared using the χ2 test.
Univariable logistic regression analysis was used to select the potential markers associated with CAV detection for inclusion in the multivariable analysis. The examined covariables included IL-33, ST2, donor age, recipient age, time from HT to blood collection, body mass index, hemoglobin, leukocyte count, erythrocyte sedimentation rate, fibrinogen, creatinine, urea, albumin, alkaline phosphatase, γ-glutamyl transpeptidase, cholesterol, creatine phosphokinase, left ventricular diastolic dimension (LVDD), left atrium dimension (LA), left ventricular ejection fraction (LVEF) and the presence of hypercholesterolemia. The relationship between variables was evaluated by Spearman’s rank correlation coefficient, and multicollinearity was evaluated by means of the tolerance and variance inflation factor. Variables with p-values < 0.2 in the univariable analysis were investigated by a multivariable logistic regression model with stepwise selection to examine the independent effect of each variable on CAV detection. The results are presented as odds ratios with 95% confidence intervals and the corresponding statistical significance.
Given the mechanism of the strong interaction between IL-33 and ST2, we also calculated the combined IL-33-ST2 score. The scores for IL-33 and ST2 were included in the logistic regression model as continuous variables, and each variable was multiplied by its corresponding β-coefficient. The final scores for combined IL-33-ST2 were calculated based on the following formula: IL-33-ST2 score = –0.0431 × IL-33 + 0.0618 × ST2. The raw score for IL-33-ST2 was multiplied by (–1) to facilitate interpretation of the results.
Receiver operator characteristic (ROC) curves were plotted, and the Youden index was used to determine the cutoff for IL-33, ST2 and for the combined IL-33-ST2 score. The utility of biomarkers for CAV detection was evaluated by calculating each area under the ROC curve (AUC), sensitivity and specificity. A p-value < 0.05 was considered statistically significant.
Results
The patients’ median age was 59.00 (45.00–66.00) years, and 74.2% were men. The median time from HT to study inclusion was 9.03 (6.02–13.01) years. Median time of the diagnosis of CAV was 5.50 (1.71–9.71) years after HT. The prevalence of CAV according to the International Society for Heart and Lung Transplantation (ISHLT) criteria in the analyzed population was 47.5%. At the time of enrollment, all patients were receiving immunosuppressive therapy with a calcineurin inhibitor and mycophenolate mofetil and were free from acute rejection (diagnosed either by echocardiography or biopsy), clinical signs of infection or symptoms of acute HF. 85.6% (n = 256) of patients received statin. During treatment, statin intolerance occurred in 43 patients (37 had muscle symptoms, and 4 had hepatotoxicity). To identify CAV predictors, patients were categorized as those with CAV and those without CAV. The baseline characteristics of the study population divided into the CAV group and the non-CAV group are presented in Table I.
Table I
Baseline characteristics of the study population divided into CAV and without CAV
| Parameters | General population N = 299# | Patients without CAV N = 157 | Patients with CAV N = 142 | P-value |
|---|---|---|---|---|
| Donor’s details: | ||||
| Age [years] | 30.0 (22.00–35.00) | 29.00 (21.00–35.00) | 31.00 (24.00–36.00) | 0.043* |
| Men, % | 222 (74.2) | 116 (73.9) | 113 (79.6) | 0.25 |
| Ischemic time [min] | 190.00 (155.00–246.00) | 189.00 (155.00–243.00) | 196.50 (160.00–247.00) | 0.24 |
| Recipient’s details: | ||||
| Age [years] | 59.00 (45.00–65.00 ) | 58.00 (37.00–64.00) | 60.00 (53.00–66.00) | < 0.001* |
| Men, % | 222 (74.2) | 112 (71.3) | 110 (77.5) | 0.23 |
| Ischemic etiology of HF, % | 121 (40.5) | 61 (38.9) | 60 (42.3) | 0.59 |
| Time from HT to blood collection [year] | 9.03 (6.02–13.01) | 7.97 (5.00–10.01) | 11.55 (8.52–15.03) | < 0.001* |
| BMI [kg/m2] | 26.00 (24.00–29.00) | 25.00 (23.00–29.00) | 27.00 (25.00–30.00) | 0.001* |
| Hemoglobin [mmol/l] | 8.70 (8.10–9.40) | 8.80 (8.20–9.40) | 8.65 (8.00–9.20) | 0.17 |
| Leukocyte count [× 109/l] | 6.68 (5.72–7.98) | 6.80 (5.78–8.13) | 6.53 (5.55–7.67) | 0.15 |
| Platelets [× 109/l] | 206.00 (176.00–254.00) | 209.00 (179.00–249.00) | 203.50 (173.00–264.00) | 0.89 |
| IL-33 [ng/l] | 32.94 (21.82–69.03) | 53.09 (31.19–88.35) | 26.59 (8.75–38.08) | < 0.001* |
| ST2 [ng/ml] | 21.20 (13.50–39.76) | 15.21 (9.27–25.14) | 28.97 (18.94–75.09) | < 0.001* |
| IL-33/ST2 | 0.33 (–1.13–1.22) | 0.95 (0.37–2.71) | –0.99 (–3.35–0.05) | < 0.001* |
| ESR [mm/h] | 19.00 (13.00–28.00) | 18.00 (11.00–25.00) | 22.00 (14.00–36.00) | 0.005* |
| Fibrinogen [mg/dl] | 371.00 (313.00–445.00) | 345.00 (301.00–413.00) | 390.50 (328.00–469.00) | < 0.001* |
| Creatinine [μmol/l] | 108.00 (95.00–126.00) | 106.00 (92.00–124.00) | 111.00 (97.00–129.00) | 0.029* |
| GFR [ml/min × 1.73 m²] | 58.72 (49.66–73.47) | 61.31 (51.55–77.29) | 56.93 (47.39–70.09) | 0.024* |
| Bilirubin [μmol/l] | 11.40 (7.90–15.30) | 11.40 (7.90–15.20) | 11.35 (8.00–15.60) | 0.84 |
| Urea [ mmol/l] | 8.60 (6.70–10.80) | 8.20 (6.50–10.20) | 9.20 (7.30–11.60) | 0.005* |
| Uric acid [μmol/l] | 423.00 (355.00–474.00) | 423.00 (365.00–456.00) | 423.50 (349.50–478.00) | 0.91 |
| Albumin [g/l] | 46.00 (44.00–48.00) | 47.00 (45.00–49.00) | 45.00 (43.00–48.00) | < 0.001* |
| Total protein [g/l] | 76.00 (71.00–80.00) | 77.00 (72.00–80.00) | 76.00 (71.00–80.00) | 0.29 |
| HbA1c, % | 5.90 (5.40–6.30) | 6.00 (5.50–6.30) | 5.85 (5.30–6.40) | 0.67 |
| Sodium [mmol/l] | 141.00 (140.00–144.00) | 141.00 (140.00–144.00) | 142.00 (140.00–143.00) | 0.67 |
| AST [U/l] | 24.00 (19.00–32.00) | 24.00 (20.00–32.00) | 24.00 (19.00–31.00) | 0.95 |
| ALT [U/l] | 21.00 (15.00–29.00) | 20.00 (15.00–27.00) | 22.00 (15.00–31.00) | 0.24 |
| CPK [U/l] | 121.00 (90.00–174.00) | 114.00 (90.00–148.00) | 124.00 (97.00–193.50) | 0.034* |
| ALP [U/l] | 85.00 (67.00–107.00) | 80.00 (65.00–104.00) | 93.50 (74.00–111.00) | 0.017* |
| GGTP [U/l] | 41.00 (25.00–72.00) | 35.00 (21.00–67.00) | 43.00 (26.00–78.00) | 0.030* |
| Cholesterol [mmol/l] | 4.61 (3.91–5.23) | 4.55 (3.78–5.13) | 4.75 (4.07–5.38) | 0.030* |
| LDL [mmol/l] | 2.39 (2.01–3.04) | 2.37 (1.99–3.00) | 2.42 (2.03–3.18) | 0.11 |
| LVEDD [mm] | 48.00 (45.00–52.00) | 47.00 (44.00–50.00) | 50.00 (46.00–54.00) | < 0.001* |
| LVEF, % | 55.00 (53.00–59.00) | 55.00 (54.00–60.00) | 55.00 (52.00–57.00) | 0.009* |
| LA [mm] | 47.00 (43.00–53.00) | 45.00 (42.00–50.00) | 48.00 (44.00–56.00) | < 0.001* |
| Comorbidities, %: | ||||
| Hypertension | 229 (76.6) | 120 (76.4) | 109 (76.8) | 0.95 |
| Type 2 DM | 201 (67.2) | 102 (65) | 99 (69.7) | 0.38 |
| Hypercholesterolemia | 176 (59.1) | 75 (47.8) | 101 (71.6) | < 0.001* |
| History of CMV infection | 103 (34.4) | 55 (35) | 48 (33.8) | 0.82 |
| History of acute rejection (ISHLT grade ≥ 2R) | 171 (57.2) | 86 (54.8) | 85 (59.8) | 0.38 |
| Treatment, %: | ||||
| Mycophenolate mofetil plus tacrolimus | 219 (73.2) | 123 (78.3) | 96 (67.6) | 0.036* |
| Mycophenolate mofetil plus cyclosporine | 80 (26.8) | 34 (21.7) | 46 (32.4) | |
| Statin | 256 (85.6) | 128 (81.5) | 128 (90.1) | 0.034* |
ALP – alkaline phosphatase, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BMI – body mass index, CAV – cardiac allograft vasculopathy, CMV – cytomegalovirus, CPK – creatine phosphokinase, DM – diabetes mellitus, ESR – erythrocyte sedimentation rate, GFR – glomerular filtration rate, GGTP – γ-glutamyl transpeptidase, HbA1c – hemoglobin A1c, HT – heart transplantation, LDL – low-density lipoprotein, LVEF – left ventricular ejection fraction.
The multivariable logistic regression analysis confirmed that lower IL-33 and higher ST2 concentrations, as well as older donor age, larger LVDD and longer time from HT to blood collection, were independent factors of CAV detection. The results of the univariable and multivariable logistic regression analysis for the presence of CAV are summarized in Table II.
Table II
Univariable and multivariable analysis of indicators for CAV
| Parameters | Univariable factors | Multivariable factors | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| IL-33(–) | 1.038 (1.028–1.049) | < 0.001 | 1.044 (1.029–1.059) | < 0.001 |
| ST2(+) | 1.054 (1.038 –1.069) | < 0.001 | 1.061 (1.040–1.083) | < 0.001 |
| Donor’s age(+) | 1.027 (1.003–1.051) | 0.026 | 1.046 (1.009–1.085) | 0.015 |
| Time from HT to blood collection(+) | 1.262 (1.182–1.347) | < 0.001 | 1.256 (1.151–1.371) | < 0.001 |
| BMI(+) | 1.095 (1.034–1.159) | 0.002 | ||
| ESR(+) | 1.016 (1.003–1.029) | 0.015 | ||
| Fibrinogen(+) | 1.003 (1.001–1.005) | 0.008 | ||
| Creatinine(+) | 1.011 (1.001–1.021) | 0.034 | ||
| Urea(+) | 1.084 (1.014–1.159) | 0.018 | ||
| Albumin(–) | 1.111 (1.037–1.190) | 0.003 | ||
| ALP(+) | 1.007 (1.000–1.015) | 0.06 | ||
| GGTP(+) | 1.001 (0.998–1.005) | 0.41 | ||
| Cholesterol(+) | 1.292 (1.046 –1.595) | 0.017 | ||
| CPK(+) | 1.002 (1.000–1.005) | 0.08 | ||
| LVEDD(+) | 1.117 (1.069 –1.167) | < 0.001 | 1.081 (1.016–1.149) | 0.013 |
| Hypercholesterolemia | 2.760 (1.705–4.469) | < 0.001 | ||
Abbreviations: see Table I. CI – confidence interval, OR – odds ratio.
ROC curves were generated to determine the utility of all independent factors in multivariable analysis and the combined IL-33-ST2 score for the detection of CAV. The AUCs of ST2 and IL-33 were found to be 0.784 and 0.780, respectively. The combined IL-33-ST2 score generated excellent power to detect CAV (AUC = 0.8626 (0.8220–0.9031)). The ROC curves for IL-33, ST2 and the combined IL-33-ST2 score are presented in Figure 1 A–C.
Figure 1
Receiver operating characteristics curves. A – Interleukin 33 serum concentration. B – ST 2 serum concentration. C – Combined IL-33 – ST2 score
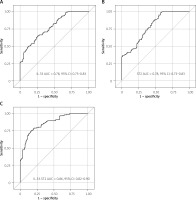
An improvement in the AUC for CAV detection was observed in the combination of IL-33 with ST2 compared to its individual components. The difference between the calculated AUCs for IL-33-ST2 and IL-33 was 0.0841 (95% CI: 0.0451–0.1230), while the difference between the AUCs for IL-33-ST2 and ST2 was 0.0787 (95% CI: 0.0317–0.1257), both being statistically significant (p < 0.001 and p = 0.001, respectively).
The AUCs for the remaining significant markers in the multivariable analysis were lower than those for IL-33/ST2 (AUC = 0.5680 for donor age, AUC = 0.7513 for time from HT to study enrollment and AUC = 0.6628 for LVDD). The differences between the AUCs for IL-33/ST2 and donor age (0.2946 (95% confidence interval (CI): 0.2206–0.3686), p < 0.001), between IL-33/ST2 and time from HT to study enrollment (0.1112 (95% CI: 0.0430–0.1794), p = 0.014), and between IL-33/ST2 and LVDD [0.1997 (95% CI: 0.1258–0.2737, p < 0.001) were statistically significant. The results obtained from the ROC analysis for the analyzed biomarkers are summarized in Table III.
Table III
Summary of ROC curve analysis for biomarkers
[i] Abbreviations: see Table I. CI – confidence interval, ROC – receiver operating characteristic.
Discussion
This single-center observational study revealed an independent association of serum IL-33 and ST2 concentrations with the presence of CAV in HT recipients. IL-33 and ST2 serum concentrations with acceptable predictive powers allow for the successful separation of CAV patients from non-CAV patients. The combined assessment of IL-33/ST2 in one model significantly increased the predictive power, sensitivity and specificity for CAV detection. Moreover, in the patients with CAV, significantly higher ST2 concentrations and significantly lower IL-33 concentrations in the peripheral blood were observed compared to the patients without CAV.
From the pathophysiological point of view, IL-33 and ST2 can be associated with the development and progression of CAV. IL-33, a member of the IL-1 cytokine family, acts by interacting with ST2 [10]. There are 2 isoforms of ST2: soluble serum circulating receptor ST2 (sST2) and transmembrane receptor ST2 ligand (ST2L). Interaction of IL-33 with the ST2L receptor in response to myocardial injury elicits a cardioprotective effect and prevents unfavorable remodeling of the heart muscle by antagonizing the action of angiotensin II and catecholamines, and the mediating mechanism is the inhibition of the nuclear factor κ-light-chain-enhancer of activated B cell transcription factor, mitogen-activated protein kinases and IκB-kinase phosphorylation [11, 16]. Furthermore, IL-33/ST2L signaling prevents fibrosis and apoptosis of cardiomyocytes and improves the function of heart muscle [11, 16]. In turn, in response to injury or stress, sST2 is produced by cardiac fibroblasts, cardiomyocytes, vessels and microcirculation endothelial cells [17]. sST2 acts as a decoy receptor for IL-33, and in pathological conditions, sST2 binds to IL-33, thereby antagonizing the same beneficial effect of the IL-33/ST2L interaction [11]. Consequently, the cardioprotective properties of IL-33 are inhibited when elevated levels of sST2 and decreased levels of IL-33 are found in blood serum, resulting in an increased risk of adverse changes in the structure and function of the heart [18]. IL-33/ST2 transmission influences the process of atherosclerotic plaque formation and regulates its hemodynamic stability. It has been proven that interferon γ produced by Th1 lymphocytes enhances the production of matrix metalloproteinases, strongly stimulates macrophages, and profoundly affects the vascular endothelium and activation of smooth muscle cells, which contributes to luminal narrowing and impaired vascular function, as well as acceleration of atherosclerotic plaque formation [19, 20]. Moreover, the excessive production of extracellular matrix metalloproteinases contributes to the destruction of the fibrous cap of atherosclerotic plaques, resulting in the formation of unstable plaques [20]. In turn, IL-33, by lowering the concentration of interferon γ (IFN-γ), prevents the activation of metalloproteinases, stabilizes atherosclerotic plaques [21] and inhibits the development of CAV. Furthermore, IL-33 induces the switch from Th1 to Th2 cell differentiation and inhibits the formation of macrophage foam cells, thus preventing atherosclerotic plaque formation [21]. Given the similarities between atherosclerosis and CAV, the above mechanisms could also be involved in the development and progression of CAV. It is believed that both acute and chronic rejection after HT result from a Th1 cell-dominated immune response, which is characterized by the massive production of several proinflammatory cytokines, including tumor necrosis factor-α and IFN-γ. In contrast, the Th2 response and type 2 cytokines such as interleukin 4 and interleukin 5 are associated with promoting graft tolerance during the progression of graft rejection [22–24]. From this point of view, IL-33, by inducing the switch from Th1 to Th2 cell transition, may prevent the development of CAV, and its low concentration in blood serum may be associated with the progression of CAV. The important activity of IL-33 within the endothelium also includes the induction of endothelial nitric oxide synthase (eNOS) activation to produce nitric oxide (NO) [23, 24]. NO is a potent vasodilator that inhibits key processes in vascular inflammation, and its absence may contribute to the acceleration of CAV [26]. In turn, IL-33 deficiency can lead to impaired eNOS/NO signaling and provoke CAV progression. Some studies have confirmed that IL-33, a novel cytokine in HT, contributes to the prevention of chronic rejection and CAV [22, 23]. Li et al., based on observations in a mouse heart transplant model, concluded that IL-33 is upregulated in allografts to limit chronic rejection by restraining the local activation of proinflammatory macrophages. Furthermore, the authors reported that in IL-33-deficient mouse cardiac transplants, accelerated vascular occlusion and subsequent fibrosis were observed. The lack of graft IL-33 causes local augmentation of proinflammatory inducible nitric oxide synthase and macrophages that accelerate the loss of the graft [22]. Brunner et al. also showed that IL-33 treatment in a murine chronic cardiac allograft rejection model promotes the Th2-type immune response, which favors myeloid-derived suppressor cells and Treg expansion, reduces B cell-dependent antibody-mediated rejection, ultimately prolongs allograft survival and prevents the development and progression of CAV [23]. In turn, in the control group without IL-33 treatment in the same model, signs of chronic allograft rejection, including perivascular leukocyte infiltrates, cardiac structural destruction, and coronary graft vasculopathy, were observed [23].
Another independent factor associated with the angiographic evidence of CAV in our study was donor age. The sensitivity of donor age in CAV detection was excellent, but its specificity was limited. Other studies have also identified donor age as a CAV predictor [24–30]. The reasons for the influence of donor age on the development of CAV remain unclear. It is speculated that allografts from older donors may be more prone to endothelial dysfunction resulting from immunological and nonimmunological interactions [27, 30]. Another explanation of this phenomenon is that elderly donor hearts have more frequent subclinical atherosclerosis that results in earlier development of clinically significant coronary disease [27–30]. These data can have clinical implications, as recipients receiving hearts from elderly donors should be more closely monitored because of the higher risk of development and progression of CAV.
Another finding of the present study was the independent association between the time from HT to study enrollment and the presence of CAV. In the CAV group, the median time from HT to study enrollment was significantly longer than in the group without CAV. These results are not surprising, as the incidence of CAV increases with time after HT. According to the registry of the ISHLT, CAV is detectable by angiography in 8% of patients within 1 year, in 30% of patients within 5 years and in 50% of patients within 10 years after HT [31].
The left ventricular (LV) diastolic dimension in M-mode echocardiography was another independent factor associated with the presence of CAV in our study group. Furthermore, the CAV2/3 group had a significantly larger LV dimension than the CAV1 group. Previous studies have shown that LV function is an important prognostic factor in patients after HT, and the presence of CAV is associated with diastolic and systolic dysfunction of the LV [32–36]. Another study also showed a significant increase in LV end systolic volume and end diastolic volume in patients with moderate and severe CAV [36]. Furthermore, stenotic microvasculopathy together with systemic hypertension and diabetes following HT may aggravate stiffness of the myocardium and contribute to dilatation of the LV, ultimately leading to LV dysfunction [5]. It is possible that the processes leading to left ventricular dilatation are more intense in CAV patients, and assessment of the change in left ventricular dimension over time may be a marker of the presence of CAV.
There are several limitations to note. As a single-center observational study, its external validation is limited. Furthermore, our population is relatively small. Larger populations may provide more statistical power to demonstrate the natural course and predictors of CAV. Prospective and multicenter studies are required to clarify the associations between our independent factors and CAV detection. In addition, other center-related factors, such as immunosuppressive treatment protocols or CAV-related study protocols, could make generalization of the findings difficult. In our study, CAV was diagnosed using CAG, and sensitivity in the detection of early CAV was limited.
According to the current standard for diagnosis of CAV we have detected the narrowing of the coronary artery lumen by CAG. The results based on the CAG may underestimate CAV prevalence in comparison with other imaging techniques, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT). Both IVUS and OCT detect early pathological changes which occur in the coronary artery vessel wall and do not compromise the lumen. Furthermore OCT produces images providing in vivo vessel histological analysis [6]. In our study, these methods of CAV diagnosis were not used, which is an important limitation of this study. Further studies on the role of IL-33 and ST2 in the detection of CAV should include more advanced techniques of imaging, such as intravascular ultrasound or optical coherence tomography. In addition, our multivariable analysis of the factors only allows for the identification of associations with CAV but not causal relations. A further limitation of the study is that blood samples were obtained at different time points after HT.
Conclusions
Our study demonstrated that lower IL-33 and higher ST2 serum concentrations, as well as older donor age, larger left ventricular diastolic dimension and longer time from HT to blood collection, are independently associated with CAV. Among the independent factors, IL-33 and ST2 have the strongest predictive power for the detection of CAV. The combined assessment of IL-33/ST2 in one model significantly increases the predictive power, sensitivity and specificity for the identification of patients with CAV. This study may have clinical implications because it provides noninvasive, low-cost, and simple indicators for CAV detection.








