Breast cancer is the most common oncological disease among women in the population of Western countries. it affects nearly 1.5 million patients per year globally [1]. Despite the increasing number of cases, early detection and diagnosis are constantly improving. Growing social awareness and greater availability of oncological procedures cause more patients to require surgical treatment. Oncological breast surgery has evolved significantly in the last two decades, and breast conserving therapy (BCT), followed by radiotherapy and oncoplastic procedures, is replacing the less frequently performed radical procedures. Particular emphasis is placed on limiting the extent of the procedure, comprehensive treatment, and individualization. the change of approach, multidisciplinary and comprehensive treatment, and better knowledge about cancer biology have all improved patients’ survival from 45–50% at the beginning of the 20th century to almost 90% today [2]. currently, a patient suffering from breast cancer is managed by a multi-profile team of specialists who form the breast cancer units (BcUs). An indispensable member of the BcU is the anesthesiologist, while acute postoperative pain is treated and supervised by the anesthesia care team. Despite the significant reduction in the extent of procedures, breast surgery is associated with significant postoperative pain and an elevated risk of developing chronic pain [3]. Given the above, optimal pain control, including choosing a proper anesthesia technique, represents the critical element of multidirectional perioperative care. the gold standard of treatment in acute postoperative pain is multimodal analgesia, an indispensable part of which is the use of infiltration techniques with local anesthetic (lA) and regional anesthesia techniques [4]. Among them, paravertebral block (PVB) has been the gold standard of care for the past decades and still remains the treatment of choice for extensive procedures [5]. Nevertheless, in recent years the development of regional anesthesia has allowed the use of new anesthetic techniques; the most important are fascial plane blocks. Among them, applications in oncological breast surgery, supported by evidence-based medicine (eBM), include PECS I and PECS II blockades, serratus plane block (SPB), and erector spinae plane block (ESPB) [5].
In the latest scientific reports, special attention is drawn to the influence of the applied anesthesia techniques (both systemic and regional) on the final oncological prognosis of patients [6]. Despite the available studies, there is a lack of well-designed, randomized, multicenter reports describing this issue.
FASCIAL PLANE BLOCKS IN BREAST SURGERY
Traditionally, nerve and plexus blocks consist of the direct deposition of LAs in the vicinity of the defined nervous structure. the subsequent diffusion of the deposited LA into the neural tissue causes reversible blockade of the voltage-gated sodium channels, inhibiting action potential formation and all-or-nothing nociceptive afferentation distal to the blockage site. the broad access to ultrasound in regional anesthesiology and the significant development of knowledge concerning its usage has led to the evolution of numerous new options for LA deposition, such as compartment or fascial plane blocks (FPBs) [7]. the concept of FPBs tends to fall outside the traditional approach to nerve and plexus blocks. the main rule classifying a given blockade in the compartment group is the drug deposition in a potential compartment delimited by two fascial plaques (interfascial). the deposition of LAs in the appropriate interfascial compartment usually takes place within the course of the nerve structures which supply the appropriate area of the body (most often the torso), and the spread of LAs causes a sufficiently wide blockade of the nerve structures, thus inducing the desired analgesic effect [8]. Another type of compartment blockade is the concept of LA supply in a given interfascial space with subsequent distribution and penetration of the medication to a dedicated area that is usually remote from the injection site and contains target nerve structures (e.g., erector spine plane block (ESPB)) [9].
Several described types of FPBs are used as an analgesic element in breast surgery. the blockades used in breast surgery that offer the best support according to the scientific evidence include [5]:
MECHANISM OF ACTION OF FASCIAL PLANE BLOCKS
Although compartment blocks have been functioning in the world of regional anesthesiology for a long time, the exact mechanism of action of all blockades has not been defined fully. the potential space between the fascial plaques, between which we deposit the LA, is filled primarily with adi-pose tissue, collagen, and the glycosaminoglycan matrix, which makes the deposited LA capable of broad transmission [10]. Secondary to drug delivery, several factors are rESPonsible for the extent of its spread along the fascial plaques. the first is the simple diffusion of the drug along a pressure gradient. At the same time, the LA can diffuse both in the fascial compartment and into the adjacent tissues (muscles) and compartments through micro-pores compacted in the fascial plates on the basis of a concentration gradient [8]. Another element is the influence of the fascia’s resting tension and the function of the adjacent muscles. elderly patients have a reduced passive tension of the fascia, which may negatively affect the extent of the developing blockage. An analogous situation is created by the use of neuromuscular blockade and reduction of the passive tension of muscles and fascia in patients under general anesthesia. Another key factor is the needle insertion path, the delivery rate, and the pressure generated during LA injection. the spread of LAs in a given fascial compartment and blockage of nerve structures contained therein represent the basic elements rESPonsible for the analgesic effect and the extent of the blockade that arises. However, Despite the blockade of specific nerve structures, the scope of the sensory block (dermatomal block distribution) is rather variable and may, in some situations, be significantly limited. Nevertheless, research shows that the final analgesic effect does not always depend on the extent of dermatomal distribution, which also suggests other mechanisms of analgesia [11, 12]. Soft tissues, muscles, fascia, and ligaments are surrounded by numerous nociceptors, which are activated only in the event of an injury and inflammation at a given site, making the tissue sensitive to pain stimuli [13]. the distribution of LAs to the adjacent, deeper tissues beyond the interfascial space (muscles, fascia, ligaments) blocks the above nociceptors, preventing their stimulation and hyperalgesia. in addition, tissue incision and surgical trauma after the blockade may facilitate the penetration of the drug into adjacent structures, favoring a broader analgesic effect. Muscle spasm and the associated focal ischemia of the muscle tissue are also a source of pain after some surgical procedures (e.g., breast surgery). therefore, blockage of motor fibers and LA diffusion to adjacent muscle structures is a potential mechanism of analgesia in some compartment blocks [14]. One such example is the PECS blockade, where mostly motor fibers are blocked, and yet the analgesic effect is preserved [15, 16]. in the case of certain compartment blockades (e.g., ESP blockade), one of the elements of analgesia, apart from the direct blockade of the nerve structures at the site of LA administration, is its distribution and diffusion through the collagen barrier (the so-called intertransverse connective tissue complex) to the paravertebral space, thereby causing paraspinal blockade [17, 18]. it should be borne in mind that only a minor amount of the total LA dose deposited can penetrate through collagen complexes into adjacent structures (e.g., into the paravertebral space, adjacent myofascial structures); hence, after most fascial blockades, including after ESPB, the final effect is inconsistent, and the extent of the blockade can vary considerably. Another essential element of any blockage is the administered dose of LA. in compartment blockades, LAs usually need to pass through a considerable distance in order to produce a clinically significant blocking effect. Hence, compartment blocks are referred to as volumetric blocks, which means that the volume of LA administered is the key factor, while the concentration matters to a smaller extent. Distinct types of nerve fibers have various levels of sensitivity to the administered LAs. the transmission of nociceptive stimuli can be attributed mainly to the smallest unmyelinated c fibers and myelinated A-delta fibers. long-acting amide LAs, most often used in FPBs, show a predilection for c fibers and, to a lesser extent, for A-delta and A-beta [19]. this is due to the greater solubility of these molecules in lipids and a higher pKa (dissociation constant), which facilitates diffusion inside the neuron and blockage of unmyelinated fibers. Hence, even using lower concentrations of long-acting amide LAs in compartment blocks, a nociceptive effect is obtained [20]. it should be noted that a significant part of the LAs administered during distribution also goes to the systemic circulation, and only a small part of it is rESPonsible for the direct blockage of nerve fibers. Based on the analgesic effects of the systemic administration of lidocaine, in the case of volume blockade, the systemic effects may also be a postulated mechanism of analgesia [21, 22]. it remains uncertain whether the long-acting amide LAs (bupivacaine, ropiva-caine) most often used in compartment blockades exhibit a similar effect of systemic analgesia [23] in the obtained plasma concentrations.
PECS I AND PECS II BLOCKADES
PECS blocks were first mentioned in 2011. Blanco described fifty patients undergoing breast surgery who underwent a new method of analgesia, which consisted of the supply of LAs in the interfascial compartment between the pectoralis major and pectoralis minor (“PECS block”), achieving satisfactory analgesic effects [24]. At present, PECS blockades include PECS I and PECS Ii, with the latter being the most widely used in breast surgery.
Figures 1 and 2 present the anatomy of the PECS blockades.
FIGURE 1
Anatomy of PECS blockades (cross section of the chest). PMM – pectoralis major muscle, PmM – pectoralis minor muscle, SM – serratus anterior muscle [Source: AnatomageTable]

FIGURE 2
Anatomy of the PECS blockades (after removal of pectoralis major muscle). PMM – pectoralis major muscle, PmM – pectoralis minor muscle, SM – serratus anterior muscle, LTN – long thoracic nerve, lateral and medial pectoral nerves [Source: AnatomageTable]

PECS I blockade is performed under ultrasound control and consists of the supply of LAs within the interfascial compartment between the two pectoral muscles, the pectoralis major and pectoralis minor, in the vicinity of the thoracobrachial artery. there are two nervous structures along the course of the aforementioned artery: the lateral thoracic nerve and the medial nerve. the lateral and medial pectoral nerves are mixed sensorimotor nerves which originate in the brachial plexus and supply both pectoral muscles. Blockade of these nerve structures is essential to achieve the analgesic effect. that said, in the case of PECS I blockade, there were no positive results in the form of the reduced intensity of postoperative pain following oncological procedures of the mammary gland, and the use of this blockade is limited to minor procedures within the pectoralis major muscle [25]. this is mainly due to the limited extent of the blockade, which only partially covers the innervation of the mammary gland.
PECS II blockade is a modification of the PECS I blockade, and the deposition of the drug occurs in two compartments, between the pectoral muscles and between the pectoralis minor and the serratus anterior. the administration of LAs between the pectoral muscles makes it possible to obtain a blockade of the pectoral nerves (similar to the PECS I blockade). if the drug is deposited between the pectoralis minor and the serratus anterior, the intercostal nerves (anterior branches of the spinal nerves) are blocked from the level of t2 to t5 and the long thoracic nerve supplying the serratus anterior. in order to obtain the optimal blockade effect due to a sufficiently wide distribution of the LAs, the needle insertion point should be at the level of the 3rd to 4th rib within the thoracic fold (Figures 3 and 4). correct positioning of the probe and location of sonoanatomical structures allows LAs to be deposited in two interfascial compartments using only one tissue puncture. there also exists a modification of the block with LA delivery below the serratus anterior [24]. However, the above modification does not affect the scope of the blockade. After proper LA deposition, the obtained range of blockade is significantly wider compared to PECS I and covers the pectoral muscles, the serratus anterior, the skin around the mammary gland, and partially the axillary fossa.
FIGURE 3
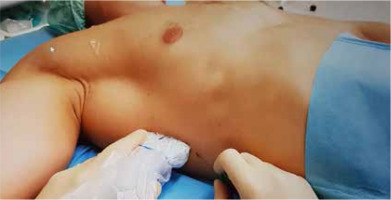
Sonoanatomy of the PECS II blockade. PMM – pectoralis major muscle, PmM – pectoralis minor muscle, SM – serratus anterior muscle, r4 – acoustic shadow of the 4th rib
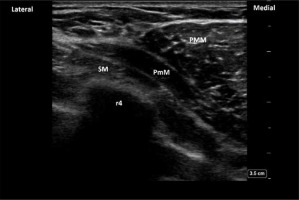
FIGURE 4
PECS II blockade. The yellow arrows indicate the place of the LA deposition (interfascial plane between two pectoralis muscles and between pectoralis minor and serratus anterior muscle). PMM – pectoralis major muscle, PmM – pectoralis minor muscle, SM – serratus anterior muscle, r4 – acoustic shadow of the 4th rib

In the light of the current evidence, PECS II blockade in patients undergoing oncological procedures of the mammary gland reduces the intraoperative and postoperative opioid requirement and reduces the postoperative intensity of pain [26, 27]. two meta-analyses also demonstrate a similar effect of PECS II blockade in the reduction of postoperative pain compared to the paravertebral blockade [28, 29]. that said, it should be noted that paravertebral blockade most often allows one to obtain a more prolonged analgesic effect which lasts more than 18 hours. PECS II blockade is therefore recommended as an alternative to PVB in the case of oncological procedures of the mammary gland but without revision of the axillary fossa. in the case of procedures extended to the axillary fossa, additional infiltration of this area should always be considered [5].
SERRATUS PLANE BLOCK
SPB blockade was described in 2013. Blanco et al. presented a description of four cases of using SPB in neuropathic pain in the thoracic region [30]. the blockade is most often performed in the fascial compartment on the anterior surface of the serratus anterior (between the serratus anterior and the latissimus dorsi). the blockade is preceded by ultrasound localization of the basic anatomical structures: the level of the fifth rib at the height of the midaxillary line, the serratus anterior, and the latissimus dorsi. Deposition of about 20 ml of lAs in the spaces between the above-mentioned muscles provides a wide range of analgesia in the anterolateroposterior region of the rib cage at the t2–t9 level. SPB modification described in later years characterizes the deposition of lAs below the serratus anterior (between the muscle and the anterior surface of the rib). At the moment, there is no convincing evidence that any version of SPB [31] is more effective in terms of blockade (Figures 5 and 6). the use of SPB blockade in breast cancer surgery, compared to placebo, reduces the intensity of pain in the postoperative period but without reducing the need for opioids [32].
FIGURE 6
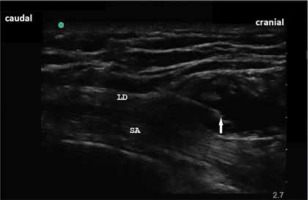
Sonoanatomy of serratus plane block. White arrow indicates the tip of the needle in the interfascial plane between latissimus dorsi muscle and serratus anterior. LD – latissimus dorsi muscle, SA – serratus anterior muscle
Additionally, the available studies comparing SPB with PVB have proven much higher effectiveness in reducing pain in the latter [33]. However, SPB may be a particularly useful additional element of analgesia in extensive oncoplastic, reconstructive breast surgery with the displacement of flaps from the latissimus dorsi muscles. this is related to the blockade of the long thoracic nerve, which innervates the above-mentioned muscular structure. this blockade may also be used in postmastectomy pain syndrome (PMPS), which may affect up to 20% of patients after mastectomy and is most often charac terized by a neuropathic character (typically located within the armpit and the anterior and lateral regions of the chest) [34]. SPB blockade as an element of multidirectional PMSP treatment may be an alternative to central blockades, PVB, or intercostal nerve blockade [31].
ERECTOR SPINAE PLANE BLOCK
ESP blockade is an ultrasound-guided compartment block used as part of multimodal analgesia, mainly after abdominal and thoracic surgery. the first description of the blockade was presented in 2016. Forero et al. presented the effect of ESP blockade in the interventional treatment of neuropathic pain after thoracic surgery [18]. the following years and scientific reports influenced the broad development of indications for the blockade and its implementation in various clinical situations. ESP blockade consists of the deposition of lAs in the fascial compartment between the erector muscle of the spine and the trans-verse process of the thoracic vertebrae. in the case of breast surgery, the t3–t5 level is recommended for LA injections. the administration of LAs at the appropriate level and in the relevant fasciomuscular compartment guarantees broad transmission of the drug in the cephalic and caudal directions. Figure 7 demonstrates the anatomy of the thoracic paraspinal region. in addition to distribution in the sagittal plane, the resting tension of the tissues (muscles and fascia) also causes the penetration of the drug towards the intercostal and medial spaces. Another postulated element is the penetration of the drug through the collagen complex separating the inter-transverse connective tissue to the paravertebral space. in the case of ESP, the paravertebral blockade effect may reduce not only the somatic but also the visceral component of pain [17, 18].
FIGURE 7
Anatomy of the thoracic paraspinal region (after removal of trapezius and rhomboideus muscles). ESM – erector spinae mus- cle, TP – transverse processes [Source: AnatomageTable]

FIGURE 8
Sonoanatomy of the erector spinae plane block. TM – tra- pezius muscle, rhomboid muscle, TP – transverse process. Yellow arrow indicates site of LA deposition

ESP blockade, as one of the youngest in the world of regional anesthesiology, can potentially be used in breast surgery and become an alternative to PVB or PECS blockade, but it requires further good-quality research and meta-analyses. the current studies suggest that the use of this blockade in surgery of the mammary gland reduces pain intensity and the need for opioids in the postoperative period [35]. these data, however, require confirmation in subsequent, ESPecially comparative studies with such blockades as PECS or PVB.
SURGICAL SITE INFILTRATION WITH LA
Local infiltration of the surgical wound using LAs, performed by the operator during the surgical procedure, constitutes one of the basic elements of multimodal analgesia after most surgical procedures [4]. it is also an established method in the vast majority of breast surgery. it should be considered in mammary gland surgery with slight and moderate tissue trauma (e.g., lumpectomy, BCT procedures). the use of local infiltration in the above cases reduces postoperative pain intensity and the need for opioids [36]. Due to the specificity and complexity of the innervation of the axillary fossa (nerve structures from both the brachial plexus and cutaneous branches of the LAteral intercostal nerves), none of the commonly used regional blockades, both compartment and PVB, fully cover this area. therefore, in the case of breast surgery involving the axillary fossa, such as sentinel lymph node dissection (SlND) or axillary lymph node dissection (AlND), additional LA infiltration of the axillary fossa should be considered in addition to the main blockage (PVB, PECS, ESP). Usage of infiltration techniques should also take account of the limited duration of the infiltration-induced effect, usually up to 6 hours after the end of surgery [5]. An alternative is the use of an implanted catheter within the operated site. Nevertheless, such a technique is not strongly reflected in the current recommendations for oncological procedures of the mammary gland [36, 37].
ADJUVANTS IN ONCOLOGICAL BREAST SURGERY
One of the methods used to extend the duration of the regional blockade is intravenous or peri-neural adjuvants. the adjuvants most often used in clinical practice are adrenaline, sodium bicarbonate, buprenorphine, tramadol, magnesium sulfate, dexamethasone, clonidine, and dexmedetomidine. Among those, the most optimal clinical effect with the best safety profile is demonstrated by dexamethasone [38]. in both large and small oncological breast surgery, intravenous administration of dexamethasone 1 hour prior to surgery significantly reduces pain intensity up to 24 hours in the postoperative period [39]. the risk of postoperative nausea and vomiting is also significantly reduced for up to 6 hours after the end of the procedure [39, 40]. One limitation in the preoperative intravenous administration of dexamethasone may be the frequent (50–70%) occurrence of severe, burning pain in the perineum, ESPecially when administered rapidly in a small volume. this can be prevented by diluting the drug in 50 ml of 0.9% Nacl and infusing it over a period of 10–15 minutes [4].
INFLUENCE OF THE TYPE OF ANESTHESIA ON THE ONCOLOGICAL PROGNOSIS
The perioperative period is associated with surgical stress, increased angiogenesis induced by the applied pharmacotherapy, and immunomodulatory effects, which may worsen the oncological prognosis and increase the risk of tumor metastasis [6]. When choosing the anesthesia technique and the type of pharmacotherapy, the anesthesiologist may be able to minimize the surgical stress and the adverse effects associated with it. there is strong evidence that the stress associated with surgical trauma is related to the risk of cancer progression in some solid neoplasms [41]. Pain secondary to tissue damage after surgical trauma and the concomitant inflammation cause activation of the hypothalamic-pituitary axis and hyperrESPonsiveness of the sympathetic system, thus contributing to the promotion of immunosuppression in the perioperative period, as evidenced by the decrease in the cytotoxic activity of natural killer (NK) cells [42]. in addition, the perioperative use of opioids in the form of morphine causes an immunomodulatory effect, impairing the function of immune cells, t lymphocytes, including NK cells, and inhibiting the production of a group of proinflammatory cytokines such as il-6 or tNF-a [43]. interestingly, this effect seems to be much less intense when synthetic opioids such as fentanyl or sufentanil are used [41]. Studies have also shown the effect of morphine on promoting neoangiogenesis by activating the receptor for the vascular endothelial cell growth factor (veGF) and thus increasing the risk of neoplastic metastases [44]. the appropriate th1/th2 lymphocyte ratio is considered to be the anti-tumor immune profile. it has been proven that the use of central blockade together with general anesthesia in oncological procedures may influence the maintenance of the correct th1/th2 ratio [41]. Recent studies also suggest that the use of central blockades in oncological operations reduces the concentration of circulating proinflammatory cyto kines and higher values of NK lymphocytes compared to the group of patients with opioid analgesia. it is commonly assumed that central blockades have a desirable and positive effect on maintaining the proper function of the immune system [41, 45]. However, in the light of current knowledge, there is not enough research to extrapolate the above data to a number of peripheral blockades.
Due to the fact that most of the current research on the impact of regional anesthesia techniques in oncological surgery on cancer prognosis and tumor metastasis comes from retrospective studies based on heterogeneous groups of patients, at the moment, there are only weak recommendations suggesting that the use of regional anesthesia techniques in the perioperative period may affect the oncological prognosis. Nonetheless, individual studies show significant differences in patients operated on, for example, for breast cancer. in the study by exadaktylos et al., patients anesthetized with PVB and total intravenous anesthesia (tivA) achieved significantly higher survival without disease recurrence and metastases compared to the group without regional anesthesia (94% vs. 77% in 36-month follow-up, rESPectively) [46]. Additionally, experimental research on breast cancer carried out in live animal models suggests that regional anesthesia reduces perioperative immunosuppression; it alleviates perioperative immunosuppression by influencing the maintenance of NK cell function [47].
CONCLUSIONS
Proper postoperative pain control is a key element of perioperative care, and breast surgery is often associated with high intensity of pain. the techniques of regional anesthesiology constitute a key element in the multidirectional approach to pain therapy. in addition to the paravertebral blockade, which is the gold standard for major oncological breast surgery, an alternative is offered by several new compartment blocks, in particular the PECS II blockade. One should also remember the use of adjuvants to improve the analgesic effect of the applied regional blockade. Among the various adjuvants used, the optimal clinical effect in the surgery of the mammary gland, and the best safety profile, are shown by dexamethasone. the subject of onco-logical prognosis in patients undergoing oncoplastic breast surgery and the anesthesia technique used are also important. currently, there are only weak recommendations and single studies suggesting that the use of regional anesthesiology techniques in the perioperative period may affect the oncological prognosis of patients undergoing major breast cancer surgery. this topic still requires further extensive research and strong scientific evidence.