Pain following total knee arthroplasty (TKA) and the methods of approaching it has been a topic of interest for many years. Options for pain management range from administration of non-steroidal anti-inflammatory drugs (NSAIDs), opioids, epidural analgesia, and patient-controlled analgesic modalities to numerous peripheral nerve blocks with or without indwelling catheters [1–3]. Among these, a well-established method is blockade of the femoral nerve (FNB) using long-acting local anaesthetics, which are frequently combined with an indwelling catheter to provide an even more prolonged anaes-thetic effect. In parallel, in recent years, the intra- and periarticular administration of local anaesthetics, combined with a variety of other substances such as opioids, NSAIDS, magnesium, and adrenaline (local infiltration analgesia – LIA) with the aim of reducing pain, swelling, and bleeding, has gained popula-rity [4]. The advantages proposed for both me-thods are the benefits of opioid sparing, and for the latter, lesser impairment of the quadriceps muscle strength, enabling earlier mobilization, and also an improved analgesic effect on the posterior capsule area [5].
The FNB has been implicated in the increased incidence of patient falls due to decreased quadriceps strength, but in most of the recent studies this could not be proven statistically. This is probably due to ultra-sound use providing more precise placement of local anaesthetic, allowing lower concentrations to be given. Another possibility is that the studies were underpowered for falls [6]. None of the studies that have been conducted were able to demonstrate any benefit of one method over the other in terms of opioid sparing effect and rehabilitation.
The main target of this trial was to compare the effects of FNB using an indwelling catheter and LIA in terms of function, pain intensity, analgesic requirements, side effects, and quadriceps strength under prospective and controlled conditions.
The primary endpoint of the study was the number of steps. The secondary endpoints were as follows: postoperative pain, consumption of opioids, rate of complications and side effects, and strength of the quadriceps muscle.
METHODS
This study was approved by the Ethical Committee of Hannover Medical School, Germany (Chairman Prof. Dr. Engeli, No. 7782) on 21 December 2016 and registered at the German Clinical Trials Register (DRKS00012492). We conducted a single-centre, randomized, unblinded, controlled trial in the orthopaedic clinic Diakovere Annastift in Hannover, Germany between February 2017 and March 2018. Patients aged 18–75 years undergoing elective primary unilateral TKA via a subvastus approach under general anaesthesia were included. Exclusion criteria were as follows: chronic pain, history of illicit substance abuse, psychiatric conditions, or cognitive disorders (whereby patients would be unable to follow an explanation of what the study entails or basic instructions and guidelines). Patients were also excluded if intraoperative or postoperative haemodynamic relevant bleeding had occurred. Blinding of the assessors or participants was not possible because the participants in the FNB group had the catheter in place whereas the LIA participants did not. Placement of a non-functioning catheter in the groin (in the LIA group) was declined by the Ethics Committee.
After obtaining written informed consent, participants were assigned to one of the interventions by a computerised random generator (Program R, R Foundation, https://www.R-project.org/foundation), and they were informed about the intended analgesic regimen before the commencement of anaesthesia. Both anaesthetic and orthopaedic teams consisted of experienced personnel in a clinic where TKA, either with FNB or with LIA, is routinely performed.
General anaesthesia was induced by remifenta-nil 0.5 µg kg–1 min–1 and propofol 1.5–2.5 mg kg–1 body weight. Orotracheal intubation was facilitated using 0.5 mg kg–1 atracurium. Anaesthesia was maintained using infusions of propofol at 3.5–4.5 mg kg–1 h–1 and remifentanil at 0.2–0.3 µg kg–1 min–1, and patients received pressure-controlled ventilation. Due to a temporary shortage of remifentanil in Germany, 0.3–0.5 mg fentanyl was used instead for the majority of patients. All patients received 1 g tranexamic acid IV perioperatively [7] and 2 g metamizol IV at the end of surgery. Depth of anaesthesia was monitored using bispectral index (M-BIS, Datex-Ohmeda, Helsinki, Finland), with a target value of 40–60.
For the implantation of the knee protheses, the subvastus approach was used. A cemented bicondylar prosthesis system was implanted. No drains were used. Compression stockings were put on the patients after surgery.
In the FNB group, the femoral nerve was localised at the femoral crease using a linear ultrasound probe of 8–18 MHz [8], generally at a depth of 2–4 cm. A 50-mm needle (UPK Nano line, Pajunk, Geisingen, Germany) was inserted out-of-plane, and a total of 10 ml of ropivacaine 0.75 % (75 mg) was applied perineurally. A catheter was advanced 2 to 3 cm over the needle tip and secured with a transparent plaster to allow inspection of the point of entry. This procedure was performed after induction of anaesthesia. Postoperatively, a patient-controlled pump for a continuous infusion with ropivacaine 0.2% at 6 mL h–1 (12 mg h–1) and an on-demand dosage of 6 mL (12 mg) with a lockout time of 60 min was connected to the catheter. The catheter was removed on the third postoperative day at the latest.
In the LIA group, a mixture of 100 mL of 0.2% ropivacaine (200 mg), 1 g tranexamic acid, 1 mg epinephrine, and 10 mL of magnesium gluconate (2.4 mmol), accounting for a total volume of 121 mL, was distributed to the operated area including the posterior capsule at the end of the surgical procedure, before closure of the wound [9].
Regarding the primary endpoint, all patients were equipped with a step tracking watch on the first postoperative day (Garmin, fēnix 3 Saphir HR) to record the number of steps. On day 4 the watches were collected, reset, and made available for the next participants, once data had been recorded and uploaded.
Postoperatively, all patients were extubated and monitored at the PACU until stable for transfer to the ward. Pain therapy was standardized and adopted to age and body weight. Initially, the patients received repeated boluses of 3.75 mg piritramide IV as required to reach a pain intensity of 30 mm or less on the visual analogue scale (VAS, range: 0–100 mm). Once awake and capable of oral intake, they were treated as follows: all patients up to the age of 60 years and those above 60 with a body weight over 90 kg received 10 mg oral slow-release oxycodone twice daily, while all other patients in this study received half of this dose. For the treatment of pain peaks, the FNB group of patients were instructed to self-apply a dose from the ropivacaine pump; if insufficient or if in the LIA group, they could (additionally) request 5–10 mg oxycodone in a fast release preparation up to 6 times a day. In all patients, this analgesic scheme was combined with 600 mg oral ibuprofen 3 times daily starting in the PACU, 40 mg pantoprazole daily, and prophylactic laxatives to prevent opioid-induced constipation [10].
Pain intensity, using a VAS and quadriceps strength [11] using the Janda score (Table 1), was assessed 4 hours after the end of surgery (day 0). This was repeated daily until at least day 5. Procedure-related side effects such as constipation, nausea and vomiting, pruritus, urinary retention, or tiredness and any complication related to LIA or NFB were recorded [12].
TABLE 1
Janda score
The Janda score data were recorded from a group of physiotherapists who were familiarised with the study. Remaining data were recorded exclusively by one of the authors of the study, thus reducing the risk of bias in both instances.
Differences between both methods were statistically analysed. Normality of distribution was tested by Kolmogorow-Smirnow test . The differences between median values were tested using the Mann-Whitney U-test and T-test. Categorial data were examined with the Fisher-Exact test. We used Wolfram Mathematica 12 (Wolfram Research, Oxfordshire, United Kingdom) for the calculations. A P-value below 0.05 was considered statistically significant.
For the conversion of the opioids to oral morphine equivalents, we used the following factors: Piritramide IV/SC: 0.5; Oxycodone oral: 0.65. The data were obtained from https://www.med-muenden.de/wp-content/uploads/2016/08/Opioid-Umrechnungstabelle2016_08_15.pdf.
RESULTS
Enrolment
Enrolment took place between 9 Feb 2017 and 6 March 2018. In total 230 patients were screened for eligibility. 133 of them did not meet the inclusion criteria and 47 declined to participate. A total of 50 patients were randomized (Figure 1). One patient had to be excluded from data analysis ex post because he had previously participated in the same study with the contralateral knee. Thus, we analysed a total of 49 patients of whom 25 were allocated to the FNB group and 24 to the LIA group.
DEMOGRAPHICS
The gender distribution was 58.3% (14) women and 41.6% (11) men in the LIA group, and of 60% (15) women and 40% (11) men in the FNB group. There were no relevant differences in the age distribution (median 65 years old LIA and 63 FNB) and body mass index between both groups. ASA scores were also similar, with most of the patients being ASA II. The results are summarised in Table 2.
TABLE 2
Demographics
| Variable | FNB (n = 25) | LIA (n = 25) | P-value |
|---|---|---|---|
| Age (years) | 63 | 65 | 0.249 |
| Gender (male/female) | 11/15 | 11/14 | 0.549 |
| BMI (kg m–2) | 30.10 | 29.28 | 0.553 |
| ASA I/II/III | 8/16/1 | 5/19 | 0.606 |
Mobilisation
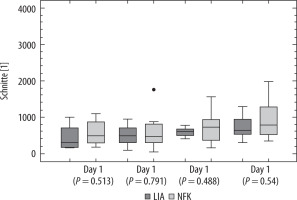
Data from the step tracker watch were obtained from a total cohort of 20 patients, 10 in each group (see discussion re: loss of data). Regarding the number of steps between days 1 to 4, there was no difference between both groups (Figure 2).
Pain scores and opioid consumption
The VAS (mean ± SD) was not different between the groups (day 0: 23 ± 17.7 vs. 32.8 ± 21.5; P = 0.101; day 1: 31.0 ± 22.3 vs. 41.7 ± 25.3; P = 0.112). Likewise, the mean opioid consumption did not differ in the FNB and LIA groups (day 0: 0.39 ± 0.17 vs. 0.50 ± 0.38; P = 0.655; day 1: 0.60 ± 0.27 vs. 0.71 ± 0.38; P = 0.406) (Figures 3 and 4). Rescue medicaments such as ibuprofen were similar in both groups.
Quadriceps strength
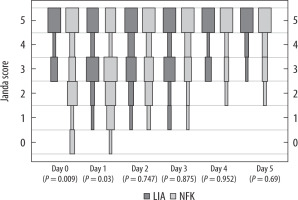
Quadriceps muscle strength, using the standardised Janda score, was significantly lower in the FNB group on day 0 (3.05 ± 1.67 vs. 4.35 ± 0.91; P = 0.009) and 1 (2.71 ± 1.57 vs. 3.67 ± 1.18; P = 0.030) but not on the following days (Figure 5).
Side effects and complications
The incidence of the side effects of opioids in both groups was low, in most cases being a tractable constipation that rapidly improved with extended coverage of laxatives (Table 3). There was only a significant difference for constipation favouring the FNB group (P = 0.009). In the FNB group one patient fell with a catheter in place, without major consequences. Neither site infections nor nerve injuries were reported in any patient from either group.
DISCUSSION
In this study we compared the effects of continuous FNB vs. LIA on postoperative function, pain, analgesic requirements, and quadriceps muscle strength, as well as side effects of the analgesic interventions in 49 patients undergoing total knee arthroplasty in general anaesthesia in a randomized, prospective, unblinded, monocentric trial. Regarding function, it is noteworthy that the number of steps taken during each day did not differ between groups despite better quadriceps contraction in the LIA group. These results, however, lack statistical significance. This could be attributable to loss of data on the step tracking watches, due to lack of compliance among patients, and some other issues which occurred. These were inhibiting factors preventing optimum data collection.
In terms of VAS and opioid consumption, no differences were observed between groups, as was seen in most other studies [6, 13]. An important point to consider is the different types and concentrations of local anaesthetics used in the studies. The same applies to LIA, where the variety of substances used, their concentrations, volume, and site of application vary widely [14–19]. Another important point is the type of anaesthesia, where in some studies spinal, general, or both were used [15, 18]. This could account mainly for the pain scores at day 0, but also for the quadriceps strength. In our study we avoided this bias, performing a total intravenous general anaesthesia in all cases.
The time at which VAS is assessed is very heterogeneous in the literature, which makes comparisons between studies difficult [6]. Furthermore, in most instances the VAS was only assessed until day 2 [6]. For that reason, we extended this period until day 5, at which point we could show that the similarity in VAS scores becomes even more evident over subsequent days.
In our study the surgical approach was the sub-vastus incision. The hypothesis is that an incision avoiding the ventral portion of the knee would produce less pain. Additionally, it should affect quadriceps strength less, favouring a faster mobility [9, 20]. By this approach we intended to define quadriceps strength that is secondary to either LIA or FNB. Further studies with subgroups with ventral and subvastus incision are needed to further investigate this hypothesis.
The Janda score was statistically superior in the LIA group at days 0 and 1, but this is probably not clinically relevant. Overall activity does not only depend on quadriceps strength; therefore, statistically significant differences are insufficient to establish whether overall function is better in the LIA group. Furthermore, at day 0 the patients were not yet mobilized, because rehabilitation began on day 1. The resulting concentrations of ropivacaine in LIA and FNB were 1.65 mg mL–1 and 7.5 mg mL–1, respectively. This difference of concentrations can be seen in all studies because the exact dose and volume for LIA is still not standardised. Additionally, the effect of additives used in LIA, which are not given with the FNB, could account for shorter or longer duration of drug effect. In addition, the heterogeneity of medicaments given in LIA in all studies [6] makes the comparison between them very difficult.
Regarding side effects (of opioids, LIA, and FNB) and falls, our study was underpowered, and so a definitive conclusion could not be reached; however, compared to other studies with higher numbers of patients, and to meta-analyses, we obtained similar results, in which no difference in falls and adverse effects were evident [6]. One patient in the FNB experienced a non-injurious fall and the incidence of opioid-related side effects, with the exception of constipation (lower incidence in the FNB group), was similarly low in both groups and not clinically relevant. As in the other studies investigating FNB and LIA, no toxic levels of ropivacaine were reached [6, 21].
There are some aspects that might favour the LIA approach: It is easier to perform, requires less equipment, and could be time saving, hence being more cost-effective. Moreover, the placement of an indwelling catheter requires daily care and surveillance by the pain nurse, which is not necessary with the LIA approach.
Moreover, there are several other approaches that are routinely performed for analgesia in TKA, e.g. adductor canal block. In this regard, the study of Borys et al. [22] (randomized, double-blinded, controlled trial) demonstrated less pain in the FNB group but faster mobility in the adductor canal nerve block group.
Our study was not without its limitations, the main one being loss of data from the step tracking watches. Secondly, the incidence of falls was low, and the study was underpowered for this complication, so a conclusion concerning this cannot be drawn. Thirdly, by using the subvastus approach in all patients studied, we avoided the element of bias, which could have been caused by differing surgical techniques. The subvastus surgical approach, however, has not been widely studied in terms of pain but mainly in terms of mobility alone. Finally, 2 significant design limitations of our trial are the lack of sample size calculation and subsequent low number of participants included in the trial, which makes it underpowered, and the open label design, which introduce a degree of bias.