In a healthy person, the sinoatrial node generates rhythmic, regular signals that lead to a contraction of the heart muscle. As early as the 18th century, it was noted that in animals the pulse rate changes with respiration, and in the mid-19th century, respiratory variability of heart rate was confirmed in humans [1]. Differences in the distance between successive RR waveforms, as recorded on electrocardiography, are a sign of cardiovascular well-being and are referred to as heart rate variability (HRV) (Figure 1).
The primary regulator of heart rate variability is the autonomic nervous system. Thanks to autonomic regulation the heart rate increases or decreases (Figure 2).
FIGURE 2
The influence of the sympathetic and parasympathetic parts of the autonomic nervous system on heart rate
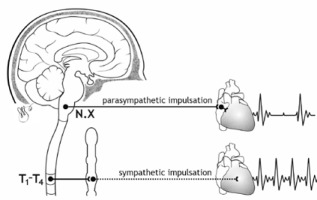
Analysis of HRV thus provides information about the interplay between the sympathetic and parasympathetic parts of the autonomic nervous system (ANS) and the potential disruption of this delicate balance, influenced by various factors [2].
Historical milestones in the study of the cardiovascular system and its variation under the influence of respiration were presented by Billman [1]. The first researchers to highlight the clinical aspects of HRV (in the foetus) were Hon and Lee [3]. In the following years, numerous researchers explored this issue and developed tools to assess the ANS, as described in detail by the aforementioned Billman [1]. HRV analysis became clinically important in the late 1980s when heart rate variability was found to be important in assessing the risk of death after myocardial infarction [4].
In 1996, the working group of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology described the standards and use of HRV in clinical practice [5].
HRV analysis is based on electrocardiographic recordings and uses 2 methods: time domain and frequency domain (spectral). The time-domain method evaluates rhythm variability in general and does not distinguish between changes specific to different frequencies. The frequency-domain method, in which random, nonrhythmic noise is ignored, allows easy distinction between major rhythmic oscillations present in HRV. For cardiovascular control in humans, the 2 main rhythmic oscillations, resulting from the parasympathetic and sympathetic activity, occurring at the frequency equal to the respiratory rate and the frequency of approx. 0.10 Hz, are of greatest importance [6].
In the time-domain method 2 types of indices are evaluated: indices calculated from the duration of consecutive R-R intervals (referred to as NN interval), which represent both components of the autonomic system (SDNN, SDANN, SDNN index), and those calculated from the differences between consecutive R-R intervals of the sinus rhythm – mainly representing the vagal tone (NN 50, pNN50, RMSSD).
The indices are defined as follows [5]:
SDNN – standard deviation of all NN intervals;
SDANN – standard deviation of the averages of NN intervals in all 5-minute segments of the entire recording;
SDANN index – mean of the standard deviation of all NN intervals for all 5-minute segments of the entire recording;
RMSSD – the square root of the mean of the sum of the squares of differences between adjacent NN intervals;
NN50 – number of pairs of adjacent NN intervals differing by more than 50 ms;
pNN50 – the number of NN50 divided by the total number of NN intervals.
Frequency analysis is defined as the evaluation of cyclic fluctuations in the R-R interval that occur at a specific frequency in a healthy individual. A fast Fourier transform (FFT) algorithm is used to sort out the chaotic total variance of heart rate variability and fit the variance to the appropriate frequency bands. The operation of the FFT can be compared to the prismatic effect of scattering light into waves of different lengths and colours. With the FFT, the variance of the R-R intervals is organized and shown as a spectrum. Specific components, charac-teristic of the cyclic variance of the R-R intervals, can be distinguished in the spectrum. Among them, the most important are the high-frequency (HF) and low-frequency (LF) power. The total power spectrum (TP) is the total variance of the signal, which represents the sum of all power frequencies. Most of the spectrum (95%) is occupied by the other 2 components: very low frequency (VLF) and ultra-low frequency (ULF). More and more is known about their significance – it is suggested that the VLF component depends on thermoregulatory mechanisms, changes induced by the renin–angiotensin– aldosterone system, and peripheral chemoreceptor activity, and the ULFs – are a consequence of diurnal oscillations [7]. Reduced variability of the VLF component is associated with arrhythmias, post-traumatic stress disorder, severe inflammation, and increased mortality [8–11].
HF changes are thought to reflect parasympathetic impulses associated with vagus nerve activity, whereas LF changes are more difficult to interpret clinically because they reflect both sympathetic and parasympathetic activity. However, in practice, LF changes can regarded as reflecting sympathetic changes. The LF/HF ratio is considered to reflect the relative and absolute changes between the sympathetic and parasympathetic components. This approach to the LF/HF ratio is simplistic, however, because it assumes that there is a sympathetic-parasympathetic balance that modulates sinus node activity in a simple way that is typical of mathematical reasoning [6].
One cannot help but mention that there are arguments against such an easy interpretation of this indicator [12].
The utility of HRV analysis in the context of chro-nic disease is well established. HRV analysis demonstrates abnormalities in the ANS activity and is correlated with increased risk of death in individuals with cardiovascular disease, diabetes, chronic kidney disease, and many other conditions [13–18].
HRV assessment has been widely used in the prediction of sudden cardiac death [19]. It is well known that chronic conditions, especially cardiovascular disease, typically characterized by reduced HRV, pose a significant burden to the anaesthetized patient and a challenge to the anaesthesiologist [20].
Therefore, it is worth trying to answer the question: is there a place for HRV analysis in anaesthesio-logy?
METHODS
A review of the Pubmed and Google Scholar databases was conducted while searching for articles focused on heart rate variability in relation to anaesthesiology. The research was performed by using the key terms “heart rate variability”, “heart rate variability anesthesiology”, “heart rate variability regional anaesthesia”, “heart rate variability subarachnoid blockade”, and “ heart rate variability epidural”.
While describing the utility of heart rate variability analysis in anaesthesia therapy, articles on adult populations were mainly used.
SPINAL BLOCK
The effects of subarachnoid anaesthesia on HRV were among the first to be described in clinical anaesthesiology. In 1995 Introna et al. [21] described the sequelae of subarachnoid anaesthesia on the ANS as “acute” isolated “withdrawal of sympathetic activity”. The blockade was achieved by administering 70–85 mg of 5% lidocaine mixed with 5% glucose. After the injection, patients were placed in the supine position. Power spectrum recordings from pre-blockade and post-blockade ECGs, comprising 150 heartbeat segments from consecutive RR wave intervals, were compared. Using an autoregressive algorithm, they found that the cephalic spread of the blockade was associated with decreased heart rate variability.
In patients in whom blockade sufficient to allow the procedure to be performed was achieved, a sig-nificant reduction in HRV occurred when sensory blockade reached the thoracic (T) level of T3–T4. For the T1–T2 level, HRV virtually disappeared – the variability in the LF component disappeared (P < 0.016). The parasympathetic activity also weakened – the HF component decreased, which the authors associate with impaired reflex responses rather than direct vagus nerve block. The LF/HF ratio, as an expression of sympathetic-parasympathetic balance, increased when the block reached T3–T4 to return to a value equal to 1 after reaching the T1–T2 level blockade. It follows that the LF/HF ratio did not permit conclusions regarding the quantitative assessment, which was obtained by using power spectrum analysis and finding a decrease in this power as an expression of suppression of rhythm variability.
Some patients required an additional dose of local anaesthetic before the procedure began because the extent of sensory blockade did not achieve surgical anaesthesia. The researchers found that in this group of patients, spectrum power was unchanged as compared to before the blockade. In the group in which the first dose was already sufficient, a decrease in spectrum power was observed.
In conclusion, the authors gathered that blockade of cardiac sympathetic impulsion, while leaving the parasympathetic pathway intact, leads to a reduction in both components of HRV – LF and HF. Based on their results, they stated that HRV analysis during sympathetic blockade may be an alternative way to assess the extent and effectiveness of the blockade.
One year earlier, HRV changes occurring during total spinal anaesthesia were described by Kimura et al. [22]. It must be emphasized that total spinal block is not used in a daily practice, occurring rather as a complication of neuraxial blockade [23]. In the paper 2 case reports of total spinal anaesthesia are described: one intentional (at the time used in Japan as a treatment for postherpetic neuralgia), and one being a complication of epidural anaesthesia. In both cases, complete subarachnoid anaesthesia was associated with a decrease in HRV; the increase in HF, as modulated by the parasympathetic system, occurred gradually and was most pronounced at the end of the block. HF decreased and heart rate and LF increased just before the return of consciousness. Although this method of anaesthesia is not used, the investigators’ description reflects the changes in autonomic system tone that occur as a result of an extensive sympathetic blockade. Based on this and similar observations, HRV analysis has found application in predicting adverse clinical consequences of a central blockade.
The older literature contains many papers arguing for the use of HRV to predict hypotension after subarachnoid blockade. These mainly concern the obstetric population. Thus, in their publications Hanss et al. [24–26] found the utility in predicting severe hypotension and used the results to implement effective preventive therapy in patients whose baseline LF/HF ratio values indicated a high risk of hypotension after the blockade.
HRV analysis in healthy pregnant women undergoing caesarean section under subarachnoid anaesthesia showed that high sympathetic tension and lack of sympathetic modulation, defined as LF/HF ratio > 2.3, found before the blockade, were independent risk factors (7.7; 95% CI: 1.04–56.6, P = 0.023) for hypotension during the subarachnoid blockade [27].
In an observational study, in women undergoing caesarean section, Bishop et al. [28] found that the LF/HF ratio, calculated from ≥ 5 minutes of ECG recording, could predict hypotension after block (P = 0.046; OR 1.478, 95% CI: 1.008–1.014), and they considered a value of 2 as the optimal cut-off point.
According to the conclusion of the systematic review by Frandsen et al. [29], the use of preoperative HRV is a promising tool for predicting hypotension after spinal anaesthesia in caesarean section.
The usefulness of HRV assessment as part of the prediction of hypotension after subarachnoid anaesthesia in diabetic patients was evaluated by Vinayagam et al. [30].
In the course of diabetes, HRV is reduced by both sympathetic and parasympathetic activity [31]. It is not surprising to find decreased HRV parameters in the studied patients. Hypotension after blockade occurred in 69%, and the indicators that differentiated the group with and without hypotension were baseline differences in SDNN and RMSSD. At the same time, these indices showed low accuracy in predicting hypotension.
A completely different method of assessing HRV was used by the group of Chamchad [32]. The authors applied a nonlinear HRV analysis – better at describing the chaos of heart rate variability – using the point correlation dimension parameter pPD2. Based on this parameter the authors were able to distinguish 2 groups of patients: the low-value (LO) group and the high-value (HI) group. The blockade resulted in a decrease in pPD2 in all subjects, but hypotension after blockade developed only in the LO group, and more ephedrine was administered in this group. Interestingly, the traditional evaluation by frequency analysis, done before the blockade was performed, showed no differences between the LO and HI groups.
In the contemporary literature, however, it is difficult to find work based on nonlinear HRV analysis in relation to anaesthesiology.
Although many papers support the clinical benefit of using HRV analysis in patients undergoing subarachnoid anaesthesia, it should be noted that some authors take a different position.
Shehata et al. [33] in a study of preeclamptic pregnant women found that temporal HRV analysis, recorded by electrical impedance, did not allow the prediction of hypotension after the blockade. However, they observed an increase in heart rate variability at 5 minutes and 15 minutes after injection compared to pre-blockade values.
A similar conclusion was reached several years earlier by Toptas et al. [34] and Kweon et al. [35]. Toptas studied the differences in effects on haemodynamics and HRV between isobaric and hyperbaric bupivacaine in a non-obstetric population. Hypotension was observed in 25% of all subjects. The subarachnoid blockade resulted in changes in HRV – a decrease in the LF/HF ratio was noted, with statistical significance found only in the heavy bupivacaine group. Similarly, the HF component increased in both groups, with a statistical difference for hyperbaric bupivacaine. LF value decreased non-statistically in both groups. The authors relate the lack of prognostic utility of HRV concerning post-block hypotension to the relatively small baseline LF/HF values in the analysed population and the small number of subjects with an LF/HF ratio > 2.5 in their study.
It is worth mentioning here that the cut-off value of 2.5 stems from previous literature reports. In the paper by Hanss et al. [26] in the group of obstetric patients with baseline LF/HF ratio > 2.5, higher doses of vasopressor had to be administered to correct hypotension compared to the group with baseline ratio < 2.5. The phenomenon limiting the changes in HRV in the study by Toptas et al. [34] may also have been, in their opinion, the block height, the median of which reached T7 in one group and T8 in the other – sympathetic simulation preserved in blockades reaching T8 may limit HRV changes. The results may also have been influenced by the fact that some patients needed vasoactive drugs to correct hypotension.
Finally, the aforementioned Kweon et al. [35] evaluated the utility of HRV in predicting hypotension after subarachnoid blockade in hypertensive patients. They found no change in LF/HF or total power spectrum in patients who exhibited hypo-tension of varying severity due to subarachnoid blockade. According to the authors, this conclusion may have been influenced by the fact that the patients studied already had impaired regulation of the autonomic system at baseline – they were hypertensive patients taking various antihypertensive drugs. Diabetes mellitus and coronary artery disease were also reported among the patients with comorbidities.
Based on the literature cited above, it appears that the use of HRV analysis in predicting hypotension after subarachnoid blockade has found greater significance in relatively healthy subjects, without chronic disease and not taking medications that affect the sympathetic nervous system.
EPIDURAL BLOCK
Epidural blockade, as a part of enhanced recovery after surgery protocols, is a very useful technique for pain treatment [36]. Thoracic block influences cardiac repolarization and results in its anti-arrhythmic action [37]. Analysis of the heart rate variability has been evaluated for years to assess changes occurring during this blockade.
Unfortunately, the interpretation of the results of heart rate variability during epidural blockade is inconclusive, as researchers have come to differing conclusions, as summarized by Veering et al. [38] as early as in the year 2000. It should be kept in mind that the researchers evaluated the effect of blockade performed at different levels – mainly thoracic and lumbar.
In a study by Fleisher et al. [39], lumbar blockade led to an increase in the LF/HF ratio, suggesting a shift in balance toward sympathetic dominance. At the same time, in the study by Hopf et al. [40] sympathetic blockade in the thoracic segment did not affect changes in LF.
In a study by Owczuk et al. [37] in a group of American Society of Anaesthesiologist (ASA) class I and II males in whom thoracic block was performed, the only independent risk factor for significant mean arterial pressure decrease was LF/HF ratio ≥ 2.5. According to the researchers, those inconsistencies in the results may be due to the uneven blockade of sympathetic fibres and the lack of blockade of sympathetic fibres innervating the adrenal medulla.
There are not many reports in the literature on the prognostic use of HRV analysis in relation to haemodynamic disturbances caused by the epidural blockade.
Deschamp et al. [41] studied HRV during epidural blockade in obstetric women. They showed decreases in sympathetic tone and increases in parasympathetic activity, whereas no static changes were found with respect to heart rate or blood pressure. Therefore, the authors could not draw any conclusions regarding the prognostic use of HRV analysis in predicting the onset of haemodynamic instability.
Another application of HRV monitoring was described by Song et al. [42]. When performing epidural blockade from sacral access in an unconscious patient, which is often the case in paediatric anaesthesia, it is not easy to confirm the effectiveness of the procedure. The use of HRV analysis as a tool to assess autonomic system tone may be helpful here, and this is what the Korean researchers focused on.
They found that decreases in time-domain HRV analysis were statistically significant (SDNN was most strongly reduced 3 minutes after the block-ade) whereas changes observed based on frequen-cy analysis were characterized by the trend towards suppression of sympathetic activity, also maximally expressed 3 minutes after performing the blockade.
In their opinion, based on HRV analysis, it is possible to confirm the efficacy of sacral epidural anaesthesia in children under general anaesthesia, although the authors emphasize the lack of objective confirmation of efficacy regarding the analgesic effect, which is the primary goal of the blockade.
PERIPHERAL BLOCKS
Peripheral blocks are essential method of pain treatment in different types of surgery [43, 44]. With regard to peripheral blockades, the literature describing the use of HRV analysis is sparse and mainly from many years ago, being mostly of historical interest.
Because of the haemodynamic abnormalities associated with brachial plexus blockade, this particular blockade has been a source of interest to investigators.
In 2013, the influence of brachial plexus blockade from axillary access and effects of the side on which the block was performed on indices of heart rate variability were described. Simeoforidou et al. [45] hypothesized that this blockade is associated with the occurrence of haemodynamic instability in approximately one-third of patients. In patients without any comorbidities, the blockade was performed with ropivacaine, without the addition of epinephrine; the need for clonidine administration during surgery was associated with exclusion from further analysis. Changes occurring under the influence of the blockade were compared, and the significance of the side on which the blockade was performed was assessed.
It was concluded that only right-sided blockade was associated with a decrease in the LF component, suggesting attenuation of sympathetic tone. Moreover, parasympathetic activity assessed by the time-domain method increased statistically significantly, and when assessed by the frequency-domain method – not significantly.
Left-sided blockade had virtually no effect on HRV parameters, except for a trend toward parasympathetic dominance, expressed as a decrease in the LF/HF ratio. The authors thus supported the hypo-thesis reported in the literature that changes in the autonomic system result from the spread of anaesthesia to the stellate ganglion on the same side, and this effect depends on the side in which the blockade was performed.
Because of the small size of the study group, they were unable to indicate whether differences between subjects with Horner syndrome or bradycardia/hypotension could be demonstrated from HRV analysis. Additionally, a decrease in the LF/HF ratio in the course of a brachial plexus blockade was also demonstrated by Frassanito et al. [46].
Several authors evaluated HRV variability in relation to stellate ganglion blockade and, among others, based on HRV changes, confirmed the dominance of the right stellate ganglion concerning the sinus node [47–49]. Only in the study by Kim et al. [49] did the left-sided blockade result in increased parasympathetic activity, whereas no changes in autonomic activity were found after the right-sided blockade.
These findings are of clinical importance because the blockade of stellate ganglion can be a useful tool in the treatment of electrical storm [50, 51].
Because changes in ANS activity toward suppression of cardiac sympathetic tone did not affect blood pressure values in the study by Koyama et al. [48], the authors concluded that right stellate ganglion blockade can be considered safe for the treatment of chronic pain.
GENERAL ANAESTHESIA
In numerous studies, it was found that general anaesthesia leads to decreased heart rate variability [52–54]. The specific changes occurring within the LF, HF, or LF/HF ratio components vary depending on the anaesthetics or opioids studied as well as combinations of different drugs [55, 56].
The practical use of HRV assessment as a prognostic tool for haemodynamic abnormalities is of clinical importance.
In the study by Fujiwara et al. [57], preoperative HRV significantly correlated with systolic blood pressure fluctuations during anaesthesia induction, whereas the relationship between HRV and heart rate changes was weak. Only the LF/HF ratio had a strong association with the occurrence of tachycardia after intubation. However, the authors emphasize that they used an unvalidated method to measure HRV.
Hypotension after induction of anaesthesia is an unfavourable prognostic condition and may occur in up to 60% of cases [58, 59].
Padley et al. [60] demonstrated that reduced preoperative rhythm variability and reduced spectral power identified patients at risk for haemodynamic instability after induction of anaesthesia with propofol and fentanyl. Hypotension was defined as a decrease in mean arterial pressure of more than 30% or a systolic blood pressure ≤ 60 mmHg. Reduced heart rate variability was associated not only with hypotension but also with bradycardia after anaesthesia induction [61].
Knüttgen et al. [62] came to a similar conclusion earlier while studying patients with diabetes. In their study, HRV parameters, except LF, were lower in those who developed hypotension.
In contrast, in Huang’s study [63], decreased spectral power, LF and HF, were independent predictors of hypotension, and the HF component determined the severity of hypotension at 15 min after endotracheal intubation.
The utility of HRV in predicting the occurrence of incident myocardial ischaemia in high-risk patients undergoing general anaesthesia was also evaluated. Reduced spectral power (< 400 ms2 Hz-1) appeared to predict the occurrence of these incidents and prolonged hospitalization [61, 64].
HRV analysis, due to the high frequency of arrhy-thmias, proved to be unsuitable for assessing changes occurring in the ANS during laryngoscopy and endotracheal intubation [65].
In a retrospective study in a group of patients without comorbidities, scheduled for so-called “major” abdominal surgery, HRV analysis was used to predict its usefulness in predicting vasoactive drug requirements, ICU, and length of hospital stay. HRV recording was done on the day before the procedure, and the patients were subjected to an orthostatic test. The patients were divided into “reactive” and “non-reactive” based on their response to the orthostatic test. “Reactive” patients were more likely to have intraoperative hypotension and bowel obstruction in the postoperative period. Also, they required longer total and intensive care unit hospitalization. The authors concluded that HRV assessment helps to identify patients with low ANS reserves and at high risk for adverse events in the postoperative period [66].
An interesting concept of the relationship between preoperative HRV and intraoperative “fluid responsiveness” is described in the editorial by Frandsen et al. [67]. They indicated that in the preoperative period, the preload responsiveness can be masked by the increased sympathetic activity, and intraoperatively, the depressed sympathetic activity causes vasoplegia and a decrease in venous return. This may lead to preload responsivity even in the normovolaemic patients. The authors state that, unfortunately, there are a lack of literature data that describe the potential pathogenic and therapeutic implications of pre- operative HRV analysis, as an assessment of autonomic nervous system function, for post-induction and intra-operative hypotension. According to the conclusion of the systematic review by the same authors [29], the use of preoperative HRV is a promising tool for predicting post-induction hypotension in abdominal surgery during general anaesthesia.
It should be noted that in most studies, heart rate variability was studied in patients unaffected by diseases causing autonomic dysfunction, and that patients taking medications affecting ANS activity were also excluded. This approach limits the applicability of the findings to the entire patient population.
Another implementation of HRV analysis during general anaesthesia is its use as a complementary tool to monitor the depth of anaesthesia. The idea behind such a monitor would be to rely on cardiorespiratory interactions and autonomic nervous system activity [68–70].
Another application of rhythm variability is to use ECG and RR segment changes as a means of assessing pain. The ANI (Analgesia Nociception Index) monitor is based on an assessment of parasympathetic tone – the higher it is, the lower the nociception (theoretically) [71].
Interestingly, HRV has also found applications outside of research and medicine, with phone apps designed to provide data on the body’s response to exertion during training and to lifestyle stresses based on HRV analysis (https://www.hrv4training.com/accessed; 10 April 2022). And although in this case the accuracy of the method may be questionable, if the anaesthesiologist had a tool that could reliably assess HRV in real time, they could theoretically use it to modify management. It should be remembered, however, that the authors of the cited studies were often unable to explicitly demonstrate the usefulness of HRV analysis in predicting the occurrence of symptoms, events, or reactions to the management used.
In conclusion, HRV and its changes in various conditions as well as the possibility of its use in correlation with the occurrence of symptoms or treatment outcomes are important from a scientific point of view as a research tool, with some, albeit limited, applicability in clinical settings. Analysis of HRV is a relatively easy way to gauge the activity of the autonomic nervous system and as such can provide the anaesthesiologist with additional datapoints, potentially useful in assessing efficacy of a blockade, adequacy of analgesia, and predicting adverse events. Interpretation of HRV can be problematic due to the multiplicity of factors that influence HRV as well as the bias in the methodo-logy used by different authors.





