Purpose
Malignant skin tumors can be differentiated into non-melanoma skin cancer (NMSC) and malignant melanoma. NMSC is the most common malignancy with increasing incidence. Two main forms of NMSC are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), and about 70% are BCC and 30% SCC. Surgery remains a standard of care, but radiation therapy is often used in non-surgical candidates, areas of poor wound healing, and in the head and neck region, if a tumor is very large or is located in an area of the skin that makes it difficult to remove with surgery. Cryotherapy is generally used to treat precancerous skin lesions, but it is rarely used as monotherapy to treat skin cancer. Although cryotherapy is not recommended as a standard treatment of NMSC due to high-rates of recurrences [1], this method is still commonly used in countries, such as Ukraine. BCC is perfectly suitable for high-dose-rate brachytherapy (HDR-BT) monotherapy since BCCs are typically locally invasive and rarely spread beyond the original tumor site. However, for some cases of SCC in our practice, external beam radiotherapy (EBRT) was required to irradiate regional lymph nodes. In order to provide guidelines for superficial and interstitial (IS) skin brachytherapy treatments for NMSC, there were series of papers published by the American Brachytherapy Society (ABS), including the ABS working group report for aspects of dosimetry and clinical practice [2], a survey of contemporary practice patterns [3], and the ABS consensus statement.
Based on the recommendations, surface custom-made mold (SC) applications were used if the depth of tumor invasion was less than 5 mm. If the depth of tumor invasion was deeper than 5 mm, IS implants were applied to treat NMSC. To increase the dose at the surface of the skin while maintaining acceptable hot spots from IS in the skin, a combination of IS and SC (IS + SC) was used.
The main purpose of this work was to review the clinical outcomes of patients with NMSC, including those who were treated previously with cryotherapy and had recurrences, with HDR-BT using SC, IS, and IS + SC treated at a single-institution.
Material and methods
Patients and data collection
From 2013 to 2018, a total of 751 patients treated for non-melanoma skin cancer with IS and SC HDR-BT at our institution were retrospectively evaluated. Age, sex, tumor type, and localization characteristics were analyzed (Table 1). Both mono-BT and post-cryotherapy BT cases were included in this study. All patients were staged according to the eighth edition of TNM staging classification for skin cancer.
Table 1
Total of 751 non-melanoma skin cancer (NMSC) cases treated from 2013 to 2018
Implant technique
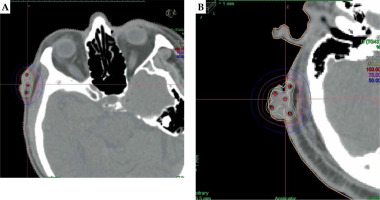
If the invasion of a tumor was less than 5 mm in depth, SC applicators were used (Fig. 1). If the invasion of a tumor was 5 mm in depth or more, IS implants were used (Figs. 2-6). In order to determine the number of catheters, spacing between them, and number of planes, Paris system [4] was applied for IS-BT implant. According to Paris system, one plane implantations should be used if the target thickness (T) is less than or equal to 12 mm. If T > 12 mm, square or triangle catheter implantations would be used. Spacing between catheters was calculated also from T parameter, including T/0.5 for two catheters in one plane, T/0.6 for more than two catheters in one plane, T/1.3 for two planes in triangle placement, and T/1.6 for two planes in square placement. Active length was calculated from lesion length parameter (L) as L/0.7. Single- and double-plane implants were used to treat 5-12 mm and 12-25 mm thick tumors, respectively. Although some implantations were sub-optimal, planning optimization could be used to meet target coverage goal and OARs constraints. Occasionally, SC implants were used together with IS to increase dose to the skin surface while treating invasion of a tumor of 5 mm deep or more.
Fig. 1
A 62-year-old patient with basal cell carcinoma (BCC), superficial treatment. A, B) On first day of treatment
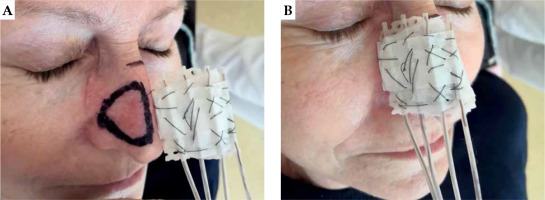
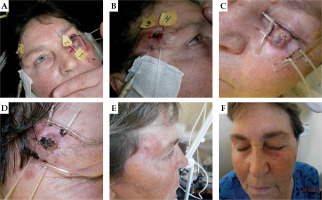
Fig. 2
A 78-year-old patient with squamous cell carcinomas (SCC). Local control of primary tumor, but lymph node failure after 1 year. A) Before BT, B) one month after BT, and (C) lymph node failure after 1 year
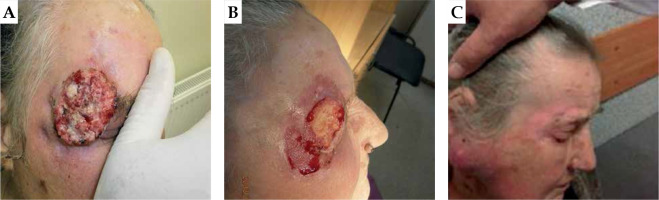
Fig. 3
A 76-year-old patient with squamous cell carcinomas (SCC). A) First day of treatment, B) one month after BT, and C) photograph taken three months after BT
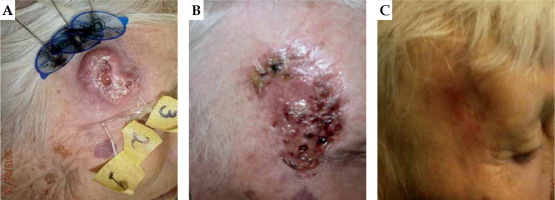
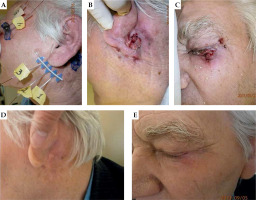
Fig. 4
A 80-year-old patient with basal cell carcinoma (BCC), interstitial treatment of two localizations. A) First day of treatment, B, C) one month after BT, and D, E) three months after BT
Planning and treatment
Clinical target volume (CTV) was determined by adding visible lesion (gross tumor volume [GTV]) and a 0.5 cm margin for BCCs or 1.0-1.5 cm margin for SCCs. Planning goal was that the prescribed dose covered CTV. For IS-BT planning, CTV was equal to planning target volume (PTV). When SC was used, a 3-4 mm margin was added to CTV to create PTV to consider uncertainty of mold placement. Organs at risk (OARs), including the lenses and mandibles for the head and neck lesions and bones for the extremities were contoured.
For BCC cases in Table 2, a total dose (TD) of 41.6 Gy in 8 fractions (5.2 Gy per fraction) was delivered. For SCC cases, 46.8 Gy in 9 fractions (5.2 Gy per fraction) was prescribed. Each fraction was delivered daily. With an α/β ratios of 6-7 Gy for BCC and 10 Gy for SCC [5-7], an equivalent dose of 2 Gy fractions (EQD2) was estimated as 58-60 Gy for both groups. Given the same α/β ratios, the biologically effective dose (BED) was 77 Gy for the group diagnosed with BCC, and 71 Gy for the SCC group.
Table 2
Treatment response, acute and late toxicities, and cosmetic results of the patients
Treatment planning was performed on computed tomography (CT) images. Forward planning with uniform dwell time loading, followed by graphical or manual optimization was used to adjust isodose distributions (Fig. 6). CTV ratio that received 150% of prescribed dose (V150) to V100 was kept below 0.45 (V150/V100 < 0.45). This constraint could be achieved when the spacing between IS catheters was about 1-1.2 cm.
Radiation dose was delivered with an HDR Elekta afterloader (MicroSelectron v3) using 192Ir radioactive source.
Endpoints
Treatment responses (local control, toxicity, and cosmetics results) were evaluated during and early after dose delivery. Treatment responses were determined according to time of tumor resorption and epithelization of skin defects occurring, and patients’ status localis (any comments or complaints during routine follow-ups). A partial response was defined in a patient, in whom the tumor was still partially present in the irradiated lesion at 1 and 3 months after treatment. Patients with partial responses had no disease-free period since the tumors were likely to grow and spread.
Local control was evaluated at 5 years after treatment. Usually, follow-up occurred at each month, every three months, and every six months after first year of treatment. Early toxicities were evaluated with skin hyperemia close to irradiated volume, swelling, and patients’ complaints. These acute effects were characterized by erythema, edema, rash dermatitis, pruritus, desquamation, and in rare cases, ulceration. These cute effects were graded as G0 for no change over baseline; G1 for follicular, faint or dull erythema, epilation, dry desquamation, or decreased sweating; G2 for tender or bright erythema, patch moist desquamation, or moderate edema; G3 for confluent, moist desquamation other than skin folds, or pitting edema; and G4 for ulceration, hemorrhage, or necrosis [8].
Late side effects appear with atrophy, pigmentation change, hair loss, telangiectasia fibrosis, and in rare cases, ulceration at six months after HDR-BT [9]. Late toxicities were graded as follows: G0 – none, G1 – slight atrophy, pigmentation change, or some hair loss, induration, G2 – patch atrophy, moderate, telangiectasia, total air loss, or induration, G3 – market atrophy, gross telangiectasia, fibrosis, G4 – ulceration or necrosis [8].
Cosmetic results were also evaluated by physicians according to RTOG grading system [8]. Slight changes in pigmentation or slight indurations were regarded as excellent, and moderate telangiectasia and fibrosis were considered good. Atrophy, gross telangiectasia, and severe induration were deemed fair, and ulceration or necrosis were considered poor cosmetic results.
Results
Patients and tumor characteristics
Between 2013 and 2018, a total of 751 non-melanoma skin cancer patients were treated with HDR-BT (Table 1). One hundred and forty-five patients (19.3%) were previously treated with cryotherapy for the same lesions. Six hundred and six patients (80.7%) were treated with exclusive BT. The median age of the patients was 71 years (range, 43-90 years). Sixty percent of the patients were males, and forty percent were females. Between the two main non-melanoma forms, BCC accounted for nearly 71%, and SCCs were about 29%. By using TNM staging classification for skin cancer, there were 298 stage I (39.7%), 434 stage II (58.2%), 12 stage III (1.6%), and 4 stage IV (0.5%) skin cancers. In this study, 90% (676 cases) of skin tumors were located in the head and neck region (6% neck, 26% scalp, 37% face, 16% nose, and 5% ear), where high conformity is extremely important. Ten percent of tumors were located on the trunk and other extremities. Out of total number of 751 patients, 225 patients (30%) were treated with SC technique, 518 patients (69%) with IS implantation, and 8 patients (1%) with IS + SC.
Clinical outcomes
After a median follow-up of 36 months (range, 12 months to 5 years), seven hundred and twenty-one patients (96%) treated with HDR-BT had complete responses to the treatment, including 513 patients in the BCC group and 205 patients in the SCC group. Also, 30 patients (4%) partially responded to the treatment, with 18 from the BCC group and 15 from the SCC group. Only 3 patients (0.4%) had local recurrences of the disease. All 3 local recurrences occurred in the BCC group. Overall, the local control rate was 95.6%. One patient from the SCC group shown in Figure 2, despite displaying good results from local treatment, was diagnosed with lymph node relapse one year later. The presence of regional lymph node metastasis was found in 3 patients from the SCC group. No patient had regional lymph node metastasis in the BCC group. The loco-regional control rates were 98.2% in the SCC group and 99.5% overall.
Toxicities
A total of 181 patients (128 patients in the BCC group and 53 patients in the SCC group) had grade 0 acute toxicity. The percentages of grade 0 toxicity in both the groups were nearly equal (24.1% in the BCC group and 24.1% in the SCC group).
A total of 570 patients (75.6%), 403 patients in the BCC group and 167 patients in the SCC group, had acute toxicity evaluated during 1- and 3-month routine check after the treatment (Table 2). According to toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (ERTOG) [8], 210 patients (28.0%) had grade 1, 351 patients (46.7%) had grade 2, and 9 patients (1.2%) had grade 3 early reaction to the radiation.
While the percentage of patients with grade 1 acute toxicity was higher in the SCC group treated with a higher prescription dose (42.7% in the SCC group vs. 21.8% in the BCC group), a larger number of patients (n = 282, 53.1%) had grade 2 early reactions in the BCC group, in which although a relatively lower dose was delivered, lesion volumes were larger in general.
Late toxicities were observed (Table 2) in 36 patients (10 cases in the BCC group and 26 cases in the SCC group). Grade 1 toxicity developed in 3.3% of patients, grade 2 toxicity occurred in 1.3% of patients, and grade 3 toxicity were observed in 0.1% of patients. All of these were detected at 6-month post-treatment or later.
Cosmetic results
Excellent cosmetic results were achieved in 79.9% of cases (600 patients: 434 in the BCC group and 166 in the SCC group; Table 2). Good cosmetic results were observed in 17.8% (134 patients: 89 and 45 from the BCC and the SCC groups, respectively), fair in 1.7% (13 patients: 8 and 5 from the BCC group and the SCC group, respectively), and poor in 0.5% of cases (4 patients from the SCC group).
Discussion
In this study, we described outcomes and toxicities of HDR interstitial and superficial treatments of 751 patients treated between 2013 and 2018. Several comparable results previously published are summarized in Table 3 [10-18]. Very good local control (LC) of 100% was reported by Svoboda et al. [10] (1995), 97.8% by Tormo et al. [11], 98% by Guix et al. [12] (2000), and 97.9% (87.2% after 5 years) by Pellizzon et al. [13] (2020). Median follow-up was 5 months and more of the first two groups, and 5 years of the second two groups. Equivalent dose in most of the studies was in a range of 60-75 Gy (EQD2), similar to our prescriptions. Although fractionation regimes varied quite rapidly from 1.8 Gy per fraction in Guix et al. [12] to 7 Gy per fraction in Tormo et al. [11], EQD2 prescriptions were very similar to our prescribed dose of 5.2 Gy per fraction. More aggressive fractionation, such as 9-10 Gy or 18-22 Gy in one fraction were reported by Svoboda et al. [10], but rarely used. At the same time, Guix et al. [12] and Svoboda et al. [10] reported excellent cosmetic results of 98% and 50%, respectively. These results were evaluated after 5 months and 5 years after last fraction of treatment, respectively.
Table 3
Summary of studies for skin cancer treatment with high-dose-rate brachytherapy (HDR-BT)
| Study (year) [Ref.] | Prescription | No. of patients/lesions | Prescription depth | Follow-up (median) | Local control | Recurrence rate | Acute toxicity | Late toxicity | Cosmetic results |
|---|---|---|---|---|---|---|---|---|---|
| Svoboda et al. (1995) [10] | 18-22 Gy one fraction, 9-10 Gy per fraction, TD = 27-30 Gy (weekly), 4 Gy per fraction, TD = 40 Gy | 76/106 | Surface | 5 and more months | 100.0% | No recurrence | G1-G2 (25.4%) | G1-G2 (5.7%) | Excellent (50.0%) Good (44.4%) Poor (5.6%) |
| Kohler-Brock et al. (1999) [18] | 5-10 Gy per fraction, TD = 30-40 Gy (one or two times per week) | 520/520 | 6-8 mm | 10 years | 92.0% | 8.0% | G1-G2 | G1-G2 | Excellent |
| Guix et al. (2000) [12] | 1.8 Gy per fraction, TD = 60-65 Gy, Boost 10-15 Gy after 3 weeks | 136/136 | 5 mm | 5 years | 98.0% | 2.0% | G1-G2 (57.6%) G4 (10.0%) | G1-G2 (0.84%) | Excellent (98.0%) Good (2.0%) |
| Pellizzon et al. (2020) [13] | Most 4 Gy per fraction, TD = 40 Gy (daily) | 71/101 | 3-5 mm | 3 years 5 years | 97.9% 87.2% | 2.1% 12.8% | G1-G2 G3 (8.9%) | G3 (3.9%) | Excellent |
| Skowronek et al. (2005) [17] | 3 Gy per fraction, TD = 48-51 Gy (BID) | 179/179 | 5 mm | 12 months | 91.1% | 8.9% | G1-G2 (87.7%) G3 (12.3%) | G1-G2 (54.0%) G3 (3.4%) | Excellent or good |
| Tormo et al. (2014) [11] | 6-7 Gy per fraction, TD = 42 Gy (twice a week) | 33/45 | 4 mm | 47 months | 97.8% | 2.2% | G1 | G1 | Excellent |
| Arenas et al. (2015) [14] | 3 Gy per fraction, TD = 54 Gy (3 times per week) | 134/134 | 5 mm | 33 months | 95.12% | 4.88% | G1-G2 (57.5%) G3 (40.3%) G4 (2.2%) | G1-G2 (3.1%) G3 (2.2%) G4 (0.8%) | Excellent/good (82.0%) Fair (13.0%) Not available (5.0%) |
| Delishaj et al. (2015) [15] | 5 Gy per fraction, TD = 40-50 Gy (2-3 times per week) | 39/57 | 4 mm | 12 months | 96.25% | No recurrence (2 lesions persisted) | G1-G2 (63.2%) | G1-G2 (19.3%) | Excellent (86.0%) Good (11.7%) Fair (2.3%) |
| Olek et al. (2018) [16] | 5 Gy per fraction, TD = 40 Gy (twice per week), 3 Gy per fraction, TD = 48 Gy (daily) | 172/273 | 3 mm | 25 months | 95.2% | 4.8% | G0 (0.4%) G1 (33.3%) G2 (48.7%) G3 (12.1%) G4 (5.1%) | G1 (2.2%) G2 (2.6%) G4 (4.4%) | |
| Present study (2022) | 5.2 Gy per fraction, TD = 41.6 Gy for BCC, TD = 46.8 Gy for SCC (daily) | 751/751 (534 BCCs, 217 SCCs) | 3-5 mm (SC), 5-25 mm (IS, IS + SC) | 36 months (12 months – 5 years) | 95.6% | 4.4% | G1 (28.0%) G2 (46.7%) G3 (1.2%) | G1 (3.3%) G2 (1.3%) G3 (0.1%) | Excellent (79.9%) Good (17.8%) Fair (1.7%) Poor (0.5%) |
Standard fractionation approach (1.8 Gy per fraction) reported by Guix et al. [12] was very beneficial from radiobiological point of view, but it was not suitable for IS technique because of a long treatment period. Also, 6-7 Gy per fraction shown by Tormo et al. [11] or higher dose (range, 9-22 Gy) per fraction reported by Svoboda et al. [10], shortened the time of treatments, but may cause more severe skin reactions or some poor cosmetic results from irradiation.
In this study, NMSC patients were treated with a 60 Gy prescription. The prescriptions reported in the published studies were about 55-65 Gy EQD2 using α/β = 6 Gy for LQ model. Pellizzon et al. [13] and Svoboda et al. [10] treated with lower prescription dose (50 Gy EQD2 with 40 Gy in 10 fractions). From a 5-year median follow-up by Pellizzon et al. [13], recurrent rate of 12.8% was much higher than in other groups (range, 2-9%). With the same prescription, Svoboda et al. [10] reported no recurrence; however, only 27 patients (30%) were treated with this prescription rate.
All groups in Table 3 reported that G1-G2 acute toxicities were usually resolved after 6 months. The same results were observed in our patients.
Although SC and IS techniques are quite different in dose normalization and hot-spot locations, our study and those published previously showed that the results are comparable for all NMSC BT.
In general, several main factors may influence local control. The factors consisted of total dose prescription, doses for fractions, previous treatment (such as EBRT or cryotherapy), tumor dimension, depth of the lesion, and histological type. It is important to perform further detail studies to establish clinical evaluations since these factors can lead to different clinical responses.