Pulmonary complications are a major cause of morbidity and mortality in children undergoing cardiac surgery [1]. Refractory respiratory failure due to various causes remains challenging to manage as conventional mechanical ventilation fails. Management of patients developing acute respiratory distress syndrome (ARDS) has been researched extensively and keeps changing. The outcome of paediatric ARDS (PARDS) is still disappointing despite implementation of several lung protective techniques [2, 3]. Around 3.2–8.4% of paediatric patients undergoing congenital heart surgery are put on extracorporeal membrane oxygenation (ECMO) support due to cardiorespiratory failure. ECMO is often less favoured than conventional therapy due to its high costs and complication rates. Moreover, the decision to institute ECMO is mainly empirical, and no universal guideline exists. Currently, the refractory respiratory failure treatment protocol, due to either PARDS or cardiorespiratory failure (e.g., due to pulmonary arterial hypertension [PAH]/ cardiogenic shock), favours implementing ECMO support in this patient population [4]. High- frequency oscillatory ventilation (HFOV) has been shown to reduce ventilator-induced lung injury in patients with cardiorespiratory failure requiring very high airway pressures on conventional ventilation. Several studies have shown improved outcomes when HFOV was used as a “rescue” therapy in such a cohort [5, 6].
Survival rates range from 40 to 80% in patients put on HFOV. The outcome of paediatric patients on HFOV is uncertain and depends on multiple factors, including the timing of the transition from conventional ventilation to HFOV and optimal ventilatory settings on HFOV [7–11].
Hence, the use of HFOV in patients developing respiratory failure after congenital heart surgery has been reviewed at our institution in terms of outcomes. The primary endpoint was to determine the rate of survival to hospital discharge in such patients following congenital heart surgery. The secondary outcome was to measure the effectiveness of HFOV in terms of improvement in respiratory parameters.
METHODS
Data collection
A retrospective review of patients after conge-nital heart surgery who were admitted to a 12-bed paediatric cardiac intensive care unit (PCICU) of a tertiary care cardiac centre was carried out with the aim of assessing the primary and secondary endpoints. A review of case files, operation room charts, and nursing charts was done of children admitted to the PCICU from October 2019 to September 2020. All patients who developed cardiorespiratory failure during the stay in the PCICU, refractory to maximal conventional mechanical ventilation, were included in the study. The patients were transitioned to HFOV or ECMO as “salvage therapy” when the maximal medical management and controlled mechanical ventilation (CMV) became ineffective. The study protocol was approved by the Institutional Ethics Committee, which waived the patient’s parental consent, due to the retrospective nature of the study.
All children on oral endotracheal intubation and CMV who failed the conventional ventilation were transitioned to HFOV. Failure of conventional ventilation was defined as oxygenation index (OI) > 40 with high positive end-expiratory pressure (PEEP) (> 10 cm H2O) and peak pressures exceeding 30 cm H2O on pressure control – synchronised intermittent mandatory ventilation (PC-SIMV), or Pplat > 28–32 cm H2O on volume control – SIMV mode, PaO2 : FiO2 (P/F) ratio < 100 and PaCO2 > 60 mmHg. Also, patients with low cardiac output syndrome due to LV, RV, or biventricular dysfunction causing severe pulmonary oedema and haemodynamic goals being achieved with the use of inotropes were considered for HFOV therapy to tide over the crisis (Table 1).
TABLE 1
Patients placed on high-frequency oscillatory ventilation (HFOV)
[i] LV – left ventricle, RV – right ventricle, PA – pulmonary artery, ASD – atrial septal defect, VSD – ventricular septal defect, PDA – patent ductus arteriosus, TAPVC – total anomalous pulmonary venous connections, ARDS – acute respiratory distress syndrome, PAH – pulmonary arterial hypertension, ECMO – extracorporeal membrane oxygenation; HFOV on POD – postoperative day on which patient was transitioned from conventional ventilation to HFOV
The details of the cases were collected from case sheets, including the aetiology of respiratory failure and postoperative course in the PCICU (Tables 1 and 2). Pulmonary parameters such as PaO2, PCO2, RR or amplitude (when on HFOV), airway pressures on ventilation (Paw), and inspired oxygen concentration (FiO2) were collected 1 hour and 3 hours prior to HFOV and 1 hour and 6 hours after initiation, and then at the time of change over to conventional ventilation or before death, whichever was applicable. Ventilatory parameters recorded on CMV were PEEP, Paw, and fraction of inspired oxygen (FiO2), and on HFOV were pressure amplitude (ΔP), mPaw, and FiO2. The respiratory parameters assessed were SpO2, RR, OI, P/F ratio, and arterial blood gas (ABG) values on mechanical ventilation (both CMV and HFOV). The primary endpoints compared included survival to discharge, total duration spent on mechanical ventilation, and length of ICU stay.
TABLE 2
Patient demographics, perioperative values and postoperative outcomes
Conventional mechanical ventilation
The postoperative ventilation of patients admitted to the PCICU was as per the institutional protocol. Depending on clinical parameters, children who developed cardiorespiratory failure and had received maximal CMV support were transitioned to HFOV or ECMO. The PC-SIMV mode was used for conventional mechanical ventilation using a GE ventilator (GE Engstrom Carestation, GE Healthcare, Finland). The conventional ventilation strategy included low tidal volumes (5–8 mL kg-1 body weight) and controlled airway pressures (Pplat less than 30 cm H2O) to avoid ventilator-induced lung injury (VILI). When patients remained hypoxic despite increasing inspiratory pressures and accepting permissive hypercapnia, with further clinical deterioration, they were transitioned to HFOV (Figure 1) [12]. The time when patients were transitioned to HFOV and the total duration that the patient continued on HFOV were noted (Table 1). Also, the total duration of mechanical ventilatory support (including both CMV and HFOV) was noted (Table 2).
High-frequency oscillatory ventilation
Initial settings of HFOV included a high FiO2 (usually 100% at the beginning) and frequency in the range 6–10 Hz. We used 8 Hz for neonates, infants, and patients weighing less than 10 kg. For children > 10 kg, 6 Hz as an initial setting was used. These settings were decided after discussion and as per existing studies [11]. The mean airway pressure (mPaw) setting was initially set at 4–5 cm H2O above the last mPaw on CMV and then adjusted, targeting a saturation of 88–92% with PaO2 of more than 60 mmHg. To achieve adequate lung volume, a recruitment manoeuvre was performed before the transition. The amplitude was titrated to visualise the vibrations from below the umbilicus to mid-thighs. Patients were weaned gradually once improvement was clinically observed. Weaning was done with gradual tapering of mPaw and oxygen concentration. The transition back to CMV was considered once the following settings were reached: FiO2 < 50%, mPaw 10–20 cm H2O, and amplitude of oscillation < 30 cm H2O with admissible results of arterial blood gas (ABG) results.
Sedation
Patients on mechanical ventilation were sedated and paralysed as per the institutional protocol (tit-ration was performed according to the Penn State Sedation Scale) [13]. We employed continuous infusion of intravenous midazolam and fentanyl with or without vecuronium. ABGs were performed every 4–6 hours as per the clinical scenario. Haemodynamic management was performed according to the Surviving Sepsis Campaign Guidelines [14].
Statistical analysis
The distribution of the continuous data was tested with the Kolmogorov-Smirnov 1-sample test. Continuous data were reported as the mean ± standard deviation or median with interquartile range, and dichotomous data were expressed as numbers and percentages. Comparison between the groups was carried out using unpaired Student’s t-test or c2 contingency tables. Mixed factor repeated measures ANOVA with Tukey’s correction was used to find any significant impact of the use of HFOV on factors such as arterial pH, PaO2, PaCO2, HCO3, and FiO2, airway pressures and respiratory rate/amplitude during mechanical ventilation and SpO2 at different periods. Statistical analysis was performed using SPSS software (IBM SPSS Statistics version 21, Chicago IL, USA) with a P-value < 0.05 being considered statistically significant.
RESULTS
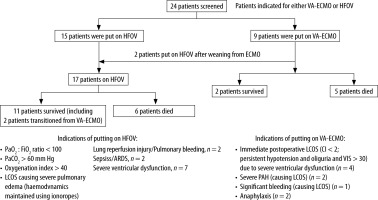
Twenty-four children with cardiorespiratory failure were candidates for a transition to either HFOV or VA-ECMO after screening all the patients who developed respiratory failure after congenital heart surgery in the study period; of these twenty-four patients, 13 (54.16%) survived (Figure 1). 15 of the 24 patients were put on HFOV while nine were treated with VA-ECMO as they fulfilled the indications for the appropriate therapies. Two patients who were initially put on ECMO were transitioned to HFOV after weaning from ECMO, making a total of 17 patients put on HFOV. Six of the 15 babies on HFOV succumbed (40%), while 5 of the nine babies on VA-ECMO (55.5%) did not survive.
Table 1 summarises the patient demographics, etiopathogenesis as preoperative pulmonary hypertension or congestive cardiac failure (CCF), intraoperative cardiopulmonary bypass (CPB) and aortic cross-clamp (AXC) periods (in minutes), CMV and ICU stay durations and post-operative complications of the patients. Both the groups of survivors and non-survivors who received HFOV therapy were comparable in demographics and perioperative variables. All patients had fulfilled the criteria for severe ARDS by the time they were initiated on HFOV.
The HFOV survivors had a median duration of 240 hours on mechanical ventilation compared to non-survivors, who spent just half the duration on CMV (approximately 123 hours). Accordingly, the survivors had a prolonged length of stay in the ICU, too (21 vs. 5.5 days; P = 0.13). Preoperative CCF and PAH were observed more amongst HFOV survivors than in the non-survivors (45.5% vs. 0%; P = 0.08). Both survivors and non-survivors had comparable intra-operative CPB and aortic cross-clamp durations (CPB: 121.2 vs. 153.3 min; P = 0.37/AXC: 87.1 vs. 88.8 min; P = 0.92) (Table 2).
Postoperative complications, though not statistically significant, were noteworthy. Non-survivors had more infliction with acute kidney injury (66.7% vs. 54.5%), more post-operative bleeding (16.7% vs. 0%), more delayed sternal closures (50% vs. 36.4%), and witnessed more rhythm disorders (16.7% vs. 9.1%) compared to the survivors. How-ever, sepsis (66.7% vs. 72.7%) and more re-intubations (16.7% vs. 45.5%) were witnessed among the survivors. None of the patients developed pneumothorax or central nervous system complications.
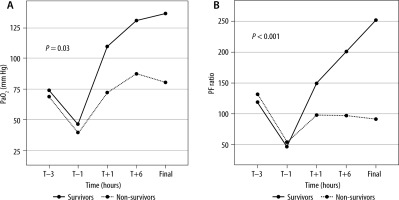
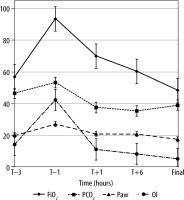
ABG results and respiratory parameters taken at scheduled timelines showed a significant improvement in the PaO2 levels of the survivors (46.5 ± 13.6 to 136.6 ± 38.7; P = 0.03) (Figure 2A). Improvement in the P/F ratio after initiation of HFOV was significant and associated with survival (46.9 ± 23.9 to 251.6 ± 107.4; P < 0.001) (Figure 2B). The remaining parameters (PCO2, RR/Amp, FiO2, Paw, and OI) also showed improvements in survivors but were not statistically significant (P > 0.05; Figure 3).
FIGURE 2
Graph showing trends of (A) PaO2 (mm Hg) and (B) PF ratio in survivors and non-survivors. P < 0.05 is significant (MANOVA test). PaO2 – partial pressure of oxygen in arterial blood, PF ratio – PaO2/FiO2 (inspired fraction of oxygen), T – initiation of high-frequency oscillatory ventilation, Final – at time of change over to conventional ventilation or before death, whichever applicable
DISCUSSION
This retrospective study presents an institutional experience of children undergoing congenital cardiac surgery who developed respiratory failure in the immediate postoperative period and were offered treatment with HFOV. Owing to high positive airway pressures during CMV, such patients can experience cardiorespiratory failure after the surgery. The advantage of HFOV lies in its continuous distending pressure that keeps the lung recruited and prevents atelectasis-induced lung injury with each respiratory cycle while simultaneously avoiding deleterious cardiopulmonary interaction due to high airway pressures. Meanwhile, low tidal volume ventilation (1–4 mL kg-1) at a high frequency maintains adequate gas exchange [3]. However, one of the earliest studies to compare HFOV to CMV did not show any survival benefit in paediatric patients but did reveal significantly less dependency on oxygen support at 30 days [4]. The use of HFOV and avoidance of damaging effects of high airway pressures on haemodynamics have not been shown to translate into survival benefits in adults [15, 16]. However, HFOV can be an effective tool for lung-protective ventilation as it delivers a very small tidal volume below the dead space, diminishing the risk of atelectrauma while simultaneously providing effective pulmonary gas exchange [17]. In a randomised, multicentre trial in infants, respiratory failure therapy with HFOV shortened the mechanical ventilation duration when compared to the infants assigned to CMV, with better survival rates [18]. However, in our study HFOV was not used as a first-line treatment but rather as a rescue treatment. This was chosen because it was a relatively new mode of ventilation whose safety and effectiveness had not been fully evaluated at our centre in this cohort.
The P/F ratio is considered conventionally an ideal tool to measure pulmonary dysfunction, especially in patients on mechanical ventilation. However, it is independent of the mean airway pressure during mechanical ventilation. In contrast, the OI is considered to be a better index to assess the severity and guide the treatment of hypoxic respiratory failure in children [19–21]. All the respiratory parameters studied showed significant improvement in the first few hours after initiation of HFOV, wherein oxygen indices improved while airway pressures decreased. In our study, survivors and non-survivors had similar gas exchange parameters during CMV, but these values became significantly better once the patient was transitioned to HFOV. The survivors showed an improvement in PaO2 along with an increase in the P/F ratio on initiation of HFOV (Figures 2 and 3). They continued with sustained improvement until significant recovery and shifting back to CMV. Thus, based on the PaO2 and P/F ratio, the response to HFOV could help identify potential survivors within the first 60 min. It should be noted that the improvement in the OI was not statistically significant. It was noteworthy that the survivors achieved an OI < 10 and P/F ratio of more than 200 within 6 hours of initiating the HFOV, while non-survivors continued to deteriorate or did not improve significantly. This aspect needs to be evaluated in further studies as it may help the physician to decide on whether to continue with HFOV or employ other rescue therapies such as ECMO in patients who are non-responders to HFOV. The findings of this study are in agreement with the previous studies, which found a positive association between improved oxygenation parameters and survival [22–24].
FIGURE 3
Graph illustrates various parameters before and after initiation of high-frequency oscillatory ventilation (HFOV) (T) and ‘final’ at the end of HFOV in survivors: FiO2 (mean delivered fraction of inspired oxygen, %). PaCO2 – mean arterial partial pressure of carbon dioxide (mm Hg), mPaw – mean airway pressure (cm H2O), and OI – oxygenation index, T – initiation of HFOV, Final – at time of change over to conventional ventilation or before death, whichever applicable
The survival to hospital discharge in a cohort of patients rescued with HFOV in the present study was 54.16%. This percentage of survival is a quantum jump if compared with a recently published study with a survival rate of 23.4% published by Chattopadhyay et al. [25]. In addition, if multiorgan dysfunction and severe sepsis are superimposed on PARDS, it is associated with higher mortality (61%) [26]. Most non-survivors in our cohort succumbed to severe biventricular dysfunction, immediate post-operative LV dysfunction, or severe PAH leading to cardiac dysfunction and death. On the other hand, babies with sepsis-induced PARDS showed a remarkable recovery on HFOV.
The major limitation of this study was its retrospective nature and small sample size. Changes in the management of such cohorts were diverse and evolving. Comparison of HFOV with VA-ECMO is difficult given the diverse nature of indications of putting patients on HFOV or ECMO. It is a matter of clinical judgment on a case-to-case basis, which could also affect the interpretation. We also did not discuss the haemodynamic parameters of the patients when transitioned to rescue therapies, as both HFOV and ECMO greatly affect the haemodynamics. Our focus was on respiratory variables and the clinical outcome of this cohort.
CONCLUSIONS
This study presents our experience with HFOV as a salvage therapy in patients with refractory hypo-xaemia after cardiac surgery when conventional ventilation has failed to maintain gas exchange. In a resource-limited setting, where ECMO is not a viable option financially, HFOV may be considered a reasonable option. This study demonstrated that HFOV as a rescue therapy in patients undergoing paediatric cardiac surgery and developing respiratory failure refractory to maximal conventional ventilation was a viable option and could improve oxygenation significantly. Similar to our study, Bojan et al. reported shorter mechanical ventilation duration and ICU stays using HFOV [27]. In developing countries, where using ECMO has significant financial implications, HFOV as a rescue therapy for respiratory failure could be considered. But a more extensive prospective controlled study is required to validate this claim.