Purpose
Lung cancer ranks as the leading cause of morbidity and mortality in China [1]. Surgery is the standard option for treating localized cancers, but not all patients with operable lung cancer qualify as candidates for surgery [2]. Radiotherapy has become an important treatment possibility for patients with lung cancer at various different stages [3]. Stereotactic body radiotherapy (SBRT) enables delivery of high doses to the tumor target with rapid dose fall-off gradients, and is widely used for treating early stage lung cancer [4]. International guidelines recommend that surgery and SBRT could both be the options for stage I lung cancer [5,6].
SBRT requires more precise management to control the tumor movement, while stereotactic ablative brachytherapy (SABT) can directly deliver 192Ir source into tumors and avoid dosimetric variation caused by tumor movement [7]. Followed the inverse square law, SABT can deliver high doses to tumors and sparing overdose to organs at risk (OARs). In the present study, we added coplanar template and performed pre-SABT plan to improve the operational efficiency and accuracy of SABT.
Material and methods
Patient selection
Between January 2016 and September 2017, thirty-three patients, aged 18-80 years, with histopathologically confirmed peripheral lung cancer were selected for this dosimetric clinical trial (Chinese Clinical Trials Register No: ChiCTR-ONC-12002715, 2012-11-23). All procedures were conducted in accordance with the ethical standards of responsible committee on institutional human experimentation and with the Helsinki Declaration of 1975, as revised in 2000, and an informed consent was obtained from every patient before treatment (approved by The Affiliated Hospital Of Luhzou Medical College Ethics Committee: k201205, 2012-09-30). Patients characteristics of are presented in Table 1. GTVL of patients needed to be < 5 cm in diameter. Patients received sequential chemotherapy as deemed appropriate.
Table 1
Characteristics of the patients
Pre-SABT plan
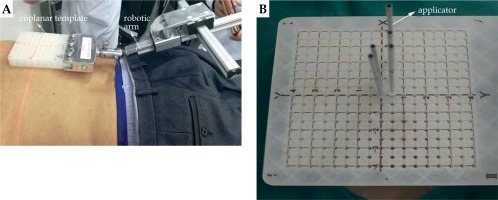
According to GTVL location, applicators were inserted at the shortest distance from GTVL, and the ribs, large vessels, heart, and other OARs were avoided, helping to decide whether the patient should be in the supine or prone position. The patient was fastened with vacuum pad, and the coplanar template was fixed by the robotic arm to cover the body surface location. The front end of the robotic arm is used to fix the coplanar template, which can rotate 360° degrees and guide the coplanar template at any angle. The coplanar template with guide holes for determining and fixing the position of the applicators is presented in Figure 1. Enhanced CT imaging was performed using a CT scanner (LightSpeed Plus 4, General Electric Company, USA), with slice thickness of 0.25 cm. Patients were breathing freely and quietly during CT examination. CT images were transferred to Oncentra 4.3 treatment planning software (Elekta Brachytherapy, Veenendaal, The Netherlands) to prepare a pre-SABT plan.
Fig. 1
A) Robotic arm used to guide the coplanar template; B) The applicator inserted into the coplanar template guide hole
CT and positron emission tomography (PET) image fusion, GTVL, and OARs were identified by a radiation oncologist. OARs included the lung, heart, esophagus, trachea, and spinal cord. The prescription dose (30 Gy) was administered to 90% of GTVL in 1 fraction. We strictly applied the prospective dose constraints for OARs according to the following principles: D1000 cm3 of the lung < 7.4 Gy, D1500 cm3 of the lung < 7 Gy; Dmax of the heart < 22 Gy, D15 cm3 of the heart < 16 Gy; D0.035 cm3 of the esophagus < 16 Gy, D5 cm3 of the esophagus < 11.9 Gy; D0.035 cm3 of the trachea < 20.2 Gy, D4 cm3 of the trachea < 10.5 Gy; Dmax of the spinal cord < 10 Gy; Dmax of skin < 26 Gy. These dose constraints were primarily based on the Radiation Therapy Oncology Group 0631, 0813, and 0915 [8,9,10].
Simulation applicators were inserted at the shortest distance from GTVL, avoiding the ribs, blood vessels, heart, and other OARs. The distance between the two simulative applicators was 0.8-1.5 cm, and the position of the simulative applicator in the coplanar template was repeatedly adjusted. In addition, repeated optimization of dose curves, using manually/graphically optimized approach of Oncentra 4.3 treatment planning software was implemented to ensure that the prescription dose of curve surrounded GTVL and OARs received a lower dose. The planner finally determined the position of the simulative applicator in coplanar template.
SABT plan
When SABT was performed, the patient position and the coplanar template position were consistent with pre-SABT plan. Local anesthesia was administered according to pre-SABT plan to determine the applicator insertion at human body surface. The applicators were inserted in GTVL along the guide hole of the coplanar template at the location determined by pre-SABT plan. The positions of applicators were adjusted according to real-time GTVL position. The diameter and length of the applicator were 0.15 cm and 20 cm, respectively. Patients were asked to avoid breathing during the applicator insertion in GTVL to reduce the possibility of applicators scratching the pleura. The number of applicators for SABT plans ranged from 1 to 5.
Patients underwent enhanced CT scans, with the same scan conditions as pre-SABT plan. CT and PET image fusion, GTVL, and OARs were delineated by a radiation oncologist to prepare a real-time SABT plan. The prescription dose of GTVL and OARs dose limits were the same as pre-SABT plan. The dose curve was optimized repeatedly for the Oncentra 4.3 treatment planning software. SABT was performed on 192Ir-source (mHDR, Elekta, Holland), with a microSelectron v3 afterloader (Elekta, Holland). Patients were breathing freely and quietly during the entire procedure. After treatment, the applicators were removed, and CT scans were obtained to identify the presence of pneumothorax, hemothorax, hemoptysis, and other complications. Patients were observed in the hospital for 24 hours. Figure 2 shows CT images, with the smallest and largest GTVL among the 33 patients studied.
Retrospective analysis of SABT plan and virtual SBRT plan
The patient’s SABT plans were revised for GTVL prescription doses of 30 Gy in 1 fraction, 46.86 Gy in 3 fractions, and 56.4 Gy in 5 fractions (all BED, 120 Gy). The prescription dose was delivered to 95% of considered GTVL. Virtual SBRT plans were designed by a sliding window intensity modulated radiotherapy (IMRT) technique using an Eclipse treatment planning system (v. 10.0; Varian), and performed on a Trilogy (Varian) linear accelerator using 6 MV photon beam. CT images obtained at pre-SABT plan were transferred to SBRT planning system to make a virtual plan. GTVL was delineated by the same radiation oncologist who identified GTVL during SABT. GTVL margins were extended outward by 0.5 cm to generate PGTVL. PGTVL prescription doses were 30 Gy in 1 fraction, 46.86 Gy in 3 fractions, and 56.4 Gy in 5 fractions, respectively (all BED, 120 Gy). The prescription dose was delivered to 95% of PGTVL.
OARs dose limits were the same as pre-SABT plan for 1 fraction. Other fractions dose constraints for OARs were applied according to the following principles: for 3 fractions, D1000 cm3 of the lung < 11.4 Gy, D1500 cm3 of the lung < 10.5 Gy; Dmax of the heart < 30 Gy, D15 cm3 of the heart < 24 Gy; D0.035 cm3 of the esophagus < 24 Gy, D5 cm3 of the esophagus < 21 Gy; D0.035 cm3 of the trachea < 30 Gy, D4 cm3 of the trachea < 15 Gy; Dmax of the spinal cord < 18 Gy; Dmax of the skin < 30 Gy. For 5 fractions: D1000 cm3 of the lung < 13.5 Gy, D1500 cm3 of the lung < 12.5 Gy; Dmax of the heart < 38 Gy, D15 cm3 of the heart < 32 Gy; D0.035 cm3 of the esophagus < 29 Gy, D5 cm3 of the esophagus < 27.5 Gy; D0.035 cm3 of the trachea < 38 Gy, D4 cm3 of the trachea < 18 Gy; Dmax of the spinal cord < 24 Gy; Dmax of the skin < 36 Gy. These dose constraints were primarily based on the Radiation Therapy Oncology Group 0631, 0813, and 0915 [8,9,10].
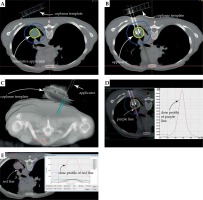
In the retrospective analysis of SABT and virtual SBRT plans, the CT images, doses, three-dimensional map, and dose profile line for one patient are shown in Figure 3. The dosimetric parameters considered for the evaluation of the treatment plan quality included the following: V100/V95/V90/V150 – percentage volume of the tumor target receiving 100%/95%/90%/150% of the prescription dose, respectively; D95% – the dose administered to 95% of the tumor target volume. Conformity index (CI) was defined using the following equation:
Fig. 3
The green, yellow, pink, and blue lines represent the 45 Gy, 30 Gy, 27 Gy, and 5 Gy isodose curves, respectively. A) CT slice images and doses for pre-SABT plan; B) CT slice images and doses for SABT plan; C) Three-dimensional map; D) Dose profile of the purple line for retrospective analysis of SABT plan; E) Dose profile of the red line for virtual SBRT plan
where TVRI is the target volume receiving the prescribed dose, TV is the target volume, and VRI is the volume of the prescribed dose.
Statistical analysis
In this study, paired t-test was used to compare the dosimetric parameters between SABT and SBRT. All statistical analysis was performed using SPSS 20.0, and p < 0.05 was considered statistically significant. Correlation analysis was performed using Pearson correlation coefficient.
Results
Safety and efficacy of SABT
The mean insertion time per applicator was 6.5 ±4.6 minutes for SABT with coplanar template assistance. The total procedure time from a patient on CT table to patient off CT table was 52.5 ±16.1 minutes. Patients did not suffer any serious complications with SABT. None of the patients had dyspnea, hemoptysis, or palpitations during the applicator insertion. Chest CT after completion of SABT revealed mild pneumothorax in two of the 33 patients; no cases of hemothorax or hemoptysis were observed. During follow-up, no radiation pneumonitis was observed. None of the patients suffered chest pain or rib fractures. The number of patients with OARs toxicity grade is summarized in Table 2. The complete response (CR) plus partial response (PR) rate for GTVL at 6-month was 100%. The local control (LC) rate for GTVL at 1-year was 96.9%. PET-CT of 2 weeks pretreatment, 6th month, and 1-year post-treatment for a patient are shown in Figure 4.
Dosimetric comparison between SABT and SBRT
Outcomes for dosimetric parameters of the lung tumor target in all patients in 1 fracture are summarized in Table 3. No statistically significant differences were noted in the lung tumor target coverage parameters, V100, V95, V90, D95%, and CI, between SABT and SBRT plans for 1, 3, and 5 fractions (all p > 0.05). However, the mean dose (Dmean) and V150 for the lung tumor target in SABT were significantly higher than those in SBRT (all p < 0.01).
Table 3
Summary of plan quality indices used for the evaluation and comparison of 1 fraction SABT and SBRT plans
For the lung in 1 fraction, Dmean, V5, V20, and V30 in terms of equivalent dose in 2 Gy (EQD2, α/β = 3), D1000 cm3, and D1500 cm3 were 2.25 Gy, 13.22%, 5.10%, 3.93%, 1.55 Gy, and 0.63 Gy, respectively, in the SABT, whereas those in the SBRT were 2.86 Gy, 17.29%, 9.87%, 7.90%, 1.62 Gy, and 0.67 Gy, respectively. For the lung in 1, 3, and 5 fractions, the D1000 cm3 and D1500 cm3 showed no statistical difference between SABT and SBRT (all p > 0.05); Dmean and V5, V20, and V30 in terms of EQD2 were all significantly lower in SABT than in SBRT (all p < 0.01). The remaining dosimetric parameters for OARs were significantly lower in SABT than in SBRT (all p < 0.01). The summaries of dosimetric parameters for OARs are shown in Figure 5.
Fig. 5
Summaries of dosimetric parameters for OARs. A, B) Summary of dosimetric parameters for the lung; C) Summary of dosimetric parameters for the heart; D) Summary of dosimetric parameters for the esophagus; E) Summary of dosimetric parameters for the trachea; F) Summary of dosimetric parameters for the skin; G) Summary of dosimetric parameters for the spinal cord

The volume of GTVL (VGTVL) surrounded by the prescribed dose had a significant correlation with irradiation time weighted by 192Ir source activity (T), the length of insertion into GTVL (L), distance (D), and number of applicators (N). The corresponding correlation coefficients (r) were 0.873, 0.690, 0.712, and 0.659, respectively (all p < 0.05). Therefore, multivariate linear regression analysis was used to fit the experimental data. The following formula was obtained:
VGTVL = Bc + B0 ×T + B1 × L + B2× D + B3× N(1)
where B0, B1, B2, and B3 were – 27.909, 0.003, 5.625, 5.128, and 3.085, respectively. The r-squared value was 0.822 (p < 0.0001) for the formula.
Discussion
A phase 3 randomized trials compared SBRT with surgery and revealed that SBRT might lead to better overall survival than surgery for operable stage I NSCLC. Lower survival after surgery might be due to comorbidities worsened by the surgical reduction of lung function [11]. SBRT requires more precise equipment to manage the tumor movement, especially respiratory movements [12,13,14]. For the SABT technique, the radioactive source and tumor are aligned in a fixed position, resulting in less movement of the applicator relative to the target tumor site even during spontaneous respiration. Therefore, we studied the safety, efficacy, and dosimetric of SABT to assess how it can provide support for the subsequent clinical application of SABT in peripheral lung cancer.
From the patient responses indicated in Table 2, SABT with coplanar template assistance was a safe treatment. For each additional 1 Gy of BED, local recurrence risk decreased by 3%, and the risk of mortality decreased by 4% [15]. If the BED is more than 100 Gy, the local control rate will be more than 80% [16,17,18,19]. In this study, BED of GTVL reached 120 Gy, so it achieved good CR, PR, and LC.
In our previous study, SABT was performed by freehand; the mean insertion time per applicator was 13.7 minutes. Inserting the applicator directly into GTVL for SABT requires skilled operators to perform the treatment and to reduce the incidence of complications generated by the procedure [20]. In present study, formula (1) shows that the length and number of applicators inserted into GTVL increase with the volume of GTVL. Thus, we added coplanar template and applied pre-SABT plan to assist the applicators insertion and reduce the impact of the surgeon’s experience on the efficacy of SABT. Pre-SABT plan enables determination of the optimal number and location of the applicators in the coplanar template, such that the dose for the target area meets a standard requirement, and spares lower dose to OARs. Coplanar template can realize one-time insertion of the applicator, reduce CT scan times, control the number and location of the applicator, and improve SABT efficiency. Therefore, the mean insertion time per applicator was 6.5 minutes, decreased by 52.6% compared to the freehand applicator insertion. We guided the applicator insertion using the coplanar template and applied the pre-SABT plan, which effectively reduced the time required for applicator to insert GTVL; the surrounding tissue may develop high pressure edema after SABT. These factors may be related to lower incidence of pneumothorax in this study. Further studies should focus on establishing standard SABT operating system to facilitate clinical applications.
Following the law of inverse squared ratio, the dose profile of SABT formed super-high dose peak for GTVL, and the dose fell more rapidly outside the tumor target area compared to that with SBRT (Figure 3D, E), resulting in a higher dose in the tumor target and a lower dose in OARs. A recent study using virtual brachytherapy plan in a single fraction to GTVL indicated comparable dosimetric parameters between brachytherapy and SBRT [21]. Our data based on realistic brachytherapy plan showed that there were no statistically significant differences in the D95% for the lung tumor target between SABT and SBRT plans (all p > 0.05). Dmean and V150 for the lung tumor target were significantly higher for SABT than for SBRT (all p < 0.01), demonstrating a significant dose escalation of SABT within lung tumor targets. For OARs, D1000 cm3 and D1500 cm3 for the lung in 1, 3, and 5 fractions showed no statistical difference between SABT and SBRT (all p > 0.05). The remaining dosimetric parameters including the heart, esophagus, trachea, and spinal cord in SABT were significantly lower than those in SBRT (all p < 0.01).
Conclusions
SABT is a safe and effective treatment for peripheral lung cancer and after assisted with coplanar template, its operational efficiency and accuracy greatly improve. Compared to SBRT, SABT can deliver a comparable prescription dosage, higher Dmean and V150 within the lung tumor target, while markedly limiting the dose to OARs. Our findings warrant generalization and application for peripheral lung cancer treatment.






