Purpose
Transperineal interstitial brachytherapy (P-ISBT) is an effective technique used for treating locally advanced cervix carcinoma (LACC) and tumors involving the vagina, including primary tumors and recurrences [1-7]. Over the past two decades, magnetic resonance imaging (MRI) has revolutionized brachytherapy (BT) planning in cervical cancer by introducing an adaptive target concept that considers primary tumor at diagnosis, and its response to external beam radiotherapy (EBRT) and concomitant chemotherapy. MRI is now the gold standard for image-guided adaptive brachytherapy (IGABT) [8], supported by development of MRI-compatible BT applicators in recent years [6].
MRI imaging provides superior soft tissue visibility compared with computed tomography (CT), allowing for better delineation of gross tumor volume (GTV) and clinical target volume (CTV), leading to smaller treatment volumes. However, there is a shortage of commercially available MRI-compatible P-ISBT applicators designed for tumors involving the vagina, which require comprehensive coverage of the entire vagina, vaginal vault, and surrounding tissues. The commercially available interstitial perineal templates are Martinez universal perineal interstitial template (MUPIT) (Elekta AB, Stockholm, Sweden) [9], Syed template (Best Medical International, Inc., Springfield, VA, USA) [10], and more recently developed Venezia applicator (Elekta AB, Stockholm, Sweden) that can adapt a perineal template to guide flexible needles. Among these, only the Syed template and Venezia applicator are MRI-compatible. However, the Venezia applicator allows perineal needle insertion, but does not permit treatment of the distal vagina. The component for vaginal treatment involves vaginal caps, which do not normally reach the lower third of the vagina.
The adoption of MRI imaging in 3D-BT has led to recommendations by the GEC-ESTRO (Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology) and the ABS (American Brachytherapy Society) for its utilization in IGABT [11-13]. Limitations of MRI-compatible commercially available interstitial applicators prompted the design and development of a mono-institutional template in 2013, known as the Template Benidorm (TB). This template, described by Rodriguez Villalba et al. [14], is a fully MRI-compatible applicator for MRI-based brachytherapy planning (Lorca Marin S.A; Murcia, Spain), combining an intra-cavitary (IC) component (vaginal cylinder with or without intra-uterine tandem), a perineal template, and titanium needles.
TB features multiple rows spaced 1.1 cm apart for the introduction of 35 straight and 16 angled (7º) titanium needles (Elekta AB, Stockholm, Sweden). It includes intra-uterine tubes angled at 15º, 30º, and 45º (Elekta AB, Stockholm, Sweden), and a 2.4 cm diameter vaginal cylinder available in various lengths to accommodate different vaginal anatomies. Plastic obturator tracks secure the needles to prevent displacement, and a double plate system ensures docking and prevents cranio-caudal needle displacement. TB combines technical advantages of MUPIT with imaging benefits of MRI-based planning, providing a stable and robust implant. Since its development, TB has been used for all P-ISBT treatments at our institution.
The aim of this work was to analyze the outcomes of patients with vaginal-involving recurrences of gynecological tumors and primary vaginal tumors treated with P-ISBT at our institution, comparing MUPIT and TB templates. Comprehensive dosimetric, clinical, and toxicity analysis of these patients was conducted, incorporating MRI in volume definition and dose-volume dosimetry.
Material and methods
Patient population
Forty-nine patients with histologically confirmed diagnosis of vaginal-involving tumors were treated with P-ISBT at our center between 2005 and 2022. Of these, 42 patients were retrospectively analyzed, as it was possible to obtain their complete follow-ups. The extent of disease was assessed in all cases using gynecological examination and diagnostic MRI. Patients included those with primary vaginal tumors, macroscopic relapse of gynecological tumors, and hysterectomized patients with positive margins or affected parametria. P-ISBT was performed using MUPIT (CT-based planning) in 18 patients (42.9%), until the development of TB in 2013. Since then, treatment has been performed exclusively with the MRI-compatible TB applicator. Therefore, 24 TB-treated patients (57.1%) were included. BT treatment was conducted by the same team, adhering to consistent treatment and planning protocols. Volumetric and dosimetric parameters as well as clinical outcomes were compared between the two applicators.
Brachytherapy procedure
A pre-implant CT (for MUPIT) or MRI (for TB) with vaginal cylinder and perineal template was performed one week before BT. Using these images, a pre-planning process defined the appropriate number, position, and depth of needles, considering 1 cm offset. Needle depth was deemed appropriate if CTV was covered with a 1 cm superior margin. For MUPIT pre-planning, a clear acetate or plastic sheet was placed over the template, and the position of each hole/cylinder was marked with ink. This sheet was then overlaid on CT axial images to determine the desired needle positions. For TB, CTV was contoured on the pre-planning MRI, and needles were virtually placed to cover CTV using a Java-based application linked to treatment planning system [15].
Applicator implantation was performed under spinal anesthesia and sedation. With the patient in the lithotomy position, a Foley tri-lumen catheter was inserted into the bladder. Obturator of the template was placed in the vagina, fitting against the vaginal vault. For MUPIT patients, this cylinder was 13 cm long. In patients with very short vaginas, the cylinder was used only to guide needle insertion and was later removed. For patients treated with TB, the cylinder length was chosen based on vaginal anatomy. Inner plate was positioned against the perineum and fixed with stitches to the perineal skin. Needles were then inserted through the template holes into the target volume, with oblique holes used for greater coverage if needed. After this, the second plate was placed to prevent longitudinal displacement of the needles. Twenty-centimeter-long needles were used: stainless steel for MUPIT and titanium for TB.
Treatment planning
Treatment planning was CT-based for patients treated with MUPIT, and exclusively MRI-based (without fusion with CT images) for those treated with TB (Figure 1). For CT-based planning, antero-posterior and lateral scout images as well as an axial sequence with a 2.5 mm slice thickness were obtained. For TB patients, MRI acquisition sequence and selected parameters with GE Signa 1.5 T (General Electric, Milwaukee, WI, USA) were as follows: T2W sequence in axial mode, repetition time = 4000-8000 ms, echo time = 66 ms, echo train length = 19 ms, slice thickness = 2.5 mm, number of excitations = 2, field of view = 200 × 200 mm2, acquisition matrix (frequency × phase) = 256 × 192, pixel size = 0.8 × 1.04 mm, and bandwidth = 97.7 Hz. The bladder was filled with 50 cc of saline solution to aid in volume definition. No rectal preparation was routinely performed.
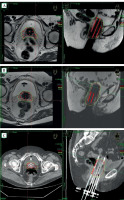
Fig. 1
Brachytherapy with TB in different situations. A) Pre-plan MRI with simulated plan, B) MRI-based treatment plan, C) Comparison of the same patient with CT-based planning
Due to characteristics of a single transperineal implant, one CTV was defined, with macroscopic disease and at-risk areas depending on each individual case. CTV included the upper and middle thirds of the vagina if only the vaginal vault was affected. In other patients, CTV comprised the entire vagina.
Implant reconstruction and treatment planning was performed in Nucletron Plato version 14.3.7 brachytherapy treatment planning system, or Oncentra MasterPlan treatment planning system version 4.3 (an Elekta company, Elekta AB, Stockholm, Sweden), as described by Richart et al. and Perez-Calatayud et al. [16-18], depending on the year of treatment. In case of TB, applicator components were incorporated into the TPS rigid applicator library to facilitate reconstruction, as described by Otal et al. [19, 20].
The applicator reconstruction was performed exclusively in the MRI T2W images set. To avoid uncertainties in the definition of needle tip, due to both artifact and slice thickness, a previously described method was employed [16-20]. This was based on: 1. The use of three small A-vitamin pellets (hyper-intense on MRI images) compressed by both applicator plates defining the central plane of the plate’s arrangement of TB [16]; 2. Free length measured outside of the external template plate; and 3. A developed library including the rigid component and interstitial part, easily adapted according to needles signals on transversal views [19].
Instead of inverse or target point-based planning, plan optimization was done through geometrical optimization, followed by manual optimization, in order to avoid excessive internal overdose volumes. Dose constraints applied to organs at risk (OARs) were based on ABS/GEC-ESTRO criteria: Rectal/sigmoid D2cc ≤ 75 Gy (EQD2) and bladder D2cc ≤ 85 Gy (EQD2) [12, 13, 21, 22]. Cumulative equivalent dose (EBRT and BT) for 2 Gy (EQD2) was calculated assuming α/β of 10 for CTV, and 3 for OARs, as recommended [12, 13].
Brachytherapy treatment was administered twice a day, with a 6-hour interval between fractions. Treatment was initially delivered using a remote afterloading MicroSelectron, and subsequently with a Flexitron HDR device (Elekta AB, Stockholm, Sweden).
Patients remained hospitalized with intravenous analgesia, antibiotic therapy, and antithrombotic measures. After administration of the final fraction, the implant was removed in the operating room under sedation, and hemostasis was always checked before patient’s discharging.
Follow-up
The first follow-up appointment was one month after the completion of treatment, and included gynecological examination and patient education on vaginal and sexual rehabilitation. Subsequent follow-ups were scheduled every 3 months for the first two years, every 6 months for up to 5 years, and then annually. Toxicity and clinical outcomes were recorded and compared in both groups of patients according to common terminology criteria for adverse events version 5.0 (CTCAE v. 5.0) [23].
Statistical study
Socio-demographic and clinical variables of participants were analyzed. Patients in both groups were matched according to their socio-demographic characteristics. Data were summarized using frequency counts, descriptive statistics, and summary tables and figures. Data analysis was performed using statistics package for social sciences (SPSS 27, IBM Inc., USA). Categorical variables were shown as frequencies and percentages. Quantitative results were represented by descriptive statistics (confidence interval, mean, and standard deviation). Chi-square test was used to compare categorical variables, expressed as n (%), and t test was employed to compare continuous variables, presented as means ± standard deviations. Kaplan-Meier method was used to analyze survival rates.
Results
The median age of patients was 59 years (range, 39-78 years). Seventeen patients (40.5%) had cervical cancer vaginal vault relapse, 15 patients (35.7%) had endometrial cancer vaginal vault relapse, 1 patient (2.4%) had a vaginal tumor relapse, and 9 patients (21.4%) had primary vaginal tumors. Ten patients (23.8%) received post-operative adjuvant treatment following local excision of tumor recurrence, and 23 patients (54.8%) received treatment for macroscopic relapse (with only a biopsy performed). The remaining 9 patients had primary vaginal tumors, and were treated with radical intent for macroscopic disease. Histology types included 18 adenocarcinomas (42.8%), 20 squamous cell carcinomas (47.6%), 1 adenosquamous carcinoma (2.4%), and 3 other histologies (7.2%). Patient and treatment characteristics are summarized in Tables 1 and 2, while Table 3 describes characteristics of re-irradiated patients.
Table 1
Patient characteristics
Table 2
Treatment characteristics
Table 3
Characteristics of re-irradiated patients
CTV included the entire length of the vagina in 26 patients (62%), and the entire circumference of the vagina in 37 patients (88%). The median number of interstitial needles was 16 in MUPIT-treated patients and 14 in TB-treated patients. The median CTV volume was 81.4 cc (range, 33.8-286.2 cc) in MUPIT, and notably smaller median volume of 47.5 cc (range, 10.0-156.4 cc) in TB patients (p = 0.01). The median EQD2 for EBRT and brachytherapy D90 CTV was 69.2 Gy (range, 27.9-88.8 Gy) in MUPIT, and 77.2 Gy (range, 31-84.3 Gy) in TB. The median rectal D2cc was 69.2 Gy (range, 23.5-82.6 Gy) in MUPIT, and 66.3 Gy (range, 16.4-75 Gy) in TB. The median bladder D2cc was 71.5 Gy (range, 23.6-90.8 Gy) in MUPIT, and 66.9 Gy (range, 18.2-78.3 Gy) in TB (Table 4).
Table 4
Dosimetric characteristics
The median follow-up for all patients was 36.5 months (range, 4-188 months). The median follow-up for MUPIT was 41.5 months (range, 14-188 months), and for TB it was 34.5 months (range, 4-98 months). Local control (LC) was 95% at 3 and 5 years. Overall survival (OS) was 77% at 3 years and 66% at 5 years. Disease-specific survival (DSS) was 81% at 3 years, and 75% at 5 years. Seven patients (17%) had lymph node recurrence, and 10 patients (24%) developed distant metastases. There were no significant differences in LC, OS, or DSS between patients treated with MUPIT or TB.
Chronic toxicity analysis is summarized in Table 5. Grade 1-2 proctitis occurred in 10 patients (24%; 8 treated with MUPIT and 2 treated with TB; p = 0.01). All patients who required argon gas treatment for proctitis had received P-ISBT with MUPIT. Grade 3 toxicity was documented in 4 patients (9.6%), 3 presented with recto-vaginal fistula (2 treated with MUPIT and 1 treated with TB), and 1 patient (2.4%) treated with MUPIT developed a vesico-recto-vaginal fistula.
Table 5
Chronic toxicity
Discussion
Primary vaginal tumors or vaginal recurrences are not uncommon in our practice. Small tumors can often be surgically removed, but due to anatomical complexity, achieving a radical oncologic procedure is not always possible. When surgery is either infeasible or incomplete, radiotherapy becomes the indicated treatment [24, 25]. Precise tumor localization and assessment of its extent, facilitated by diagnostic MRI, are crucial for patients. BT plays a key role in the radical treatment of these tumors, either as a standalone therapy or combined with EBRT. Intra-cavitary BT can be utilized if CTV depth is ≤ 5 mm, otherwise, an interstitial component is necessary. Perineal interstitial implants can be performed using a guiding template or via free-hand needle insertion, guided by vaginal and rectal touch or by ultrasound. Free-hand needle insertion is a complex, highly user-dependent technique [26, 27]. Few commercial perineal templates are MRI-compatible [6]. Traditional templates (i.e., MUPIT, Syed) use rigid needles (titanium for MRI), maintaining implant’s stability and geometry. Venezia applicator uses flexible needles, increasing the risk of deviation through inhomogeneous soft tissue in the pelvis. Local recurrences present a significant challenge, with a poorer prognosis and increased potential toxicity, as many patients receive prior radiation treatments.
A pre-plan for BT procedures was designed and published by the authors in 2017 [15], demonstrating clinical feasibility and efficiency, recently confirmed by Huang et al. [28].
The study’s limitations include its retrospective nature. Although CT and MRI images are not strictly comparable, our patient profile remained consistent over the years, with the imaging technique at the time of planning being the main difference. We followed IGABT protocols introduced in gynecological 3D-BT trials [29, 30], which helped to demonstrate a significant reduction in CTV volume. The median total EQD2 dose was 73.4 Gy (69.2 Gy for MUPIT treatments, and 77.2 Gy for TB treatments), lower than the typical dose in interstitial series compared with intra-cavitary series (EQD2, 75-80 Gy) [24, 31]. The single-implant approach with two daily fractions results in a nominally lower dose, with incomplete knowledge of radiobiological effects. Dose specification for interstitial implants varies among institutions. Limitations also arise from CT imaging use in MUPIT patients, complicating CTV definition without MRI’s soft tissue distinction, and dealing with metallic needle artifacts. Most institutions determine source activation based on silver markers indicating residual disease extent, or CTV defined at the needle perimeter [32, 33].
Our study’s 5-year overall LC results surpass those previously published, with no significant differences between patients treated with MUPIT or TB. Yoshida et al. [4] and Kotsuma et al. [34] reported 78% and 85% LC at 3 years, respectively, while Jhingran et al. [35] showed 75% at 5 years. We observed two local recurrences, both in TB-treated patients. One patient relapsed at the CTV margin, and another with carcinosarcoma, likely due to insufficient dose for this radio-resistant histology. Both patients received EBRT and P-ISBT, with total EQD2 of 68.70 Gy and 77.6 Gy, respectively. EQD2 D90 values were not predictors for local recurrence, as confirmed by other authors [24]. Despite smaller CTV volumes defined with MRI and higher doses in TB patients, there were no differences in local control. The GEC ESTRO-ABS-Canadian Brachytherapy Group (CBG) recently published target definitions for recurrent or primary vaginal cancers, defining HR-CTV and IR-CTV [25]. Previously, there were no guidelines for CTV delineation in vaginal vault recurrences. Vargo et al. [36] included disease at diagnosis in CTV limits before EBRT treatment. Lee et al. [37] defined CTV as clinically evident disease identified by examination, CT, and/or T2 MRI images at BT time, targeting a minimum of 60 Gy in the uninvolved vagina. Fokdal et al. [38] considered CTV as residual tumor at BT time, with an individual margin based on the initial tumor. Our department’s CTV delineation criteria include residual disease at BT time and disease extent at diagnosis.
Comparing OARs doses in CT-based vs. MRI-based groups, contouring accuracy in both imaging modalities is a potential limitation. Equivalents were assumed based on literature findings, with Wang et al. [39] concluding no significant differences, similar to previous studies [40-43]. Unlike intra-cavitary brachytherapy, there is little consensus on dose-volume parameters for late rectal toxicity in interstitial BT. Treatments in such patients often involved the entire vagina, with a larger proportion of rectum receiving radiation than in standard LACC treatments. Our series found no significant differences in rectal D2cc values between CT and MRI groups. Yoshida et al. [4] did not find a correlation between rectal D2cc and toxicity, with no significant difference in D2cc values between patients with or without recto-vaginal fistula formation. Lee et al. [44] reported that rectal D2cc > 61.8 Gy is associated with a 10% increase in risk of grade 2 or higher rectal toxicity. Chopra et al. [45] concluded that the 2-cc rectal dose for interstitial BT should be lower than for intra-cavitary brachytherapy. In the current study, six patients (14%) had rectal D2cc > 75 Gy, all treated with MUPIT. This is partly due to the disease extent and proximity of needles to the rectum. Only one of these six patients developed a vesico-recto-vaginal fistula (rectal D2cc = 82.6 Gy, bladder D2cc = 90.8 Gy), with a CTV volume no larger than the median for MUPIT (70.80 cc; MUPIT median volume = 81.4 cc).
TB significantly reduces CTV volume (as defined by MRI) resulting in higher D90% values, and a statistically significant (p < 0.01) reduction in grade 2 radiation proctitis and the need for argon gas treatments. Our grade 3 toxicity rates are comparable to those published by other authors. Beriwal et al. [1] reported 7% of grade 3-4 toxicity in 16 patients treated with free-hand implantation interstitial BT ± EBRT for primary LACC or vaginal cancers. Goodman et al. [5] reported 10.4% of grade 3-4 toxicity in 67 patients treated with EBRT and ISBT for primary vaginal tumors. Mulye et al. [46] described 6.7% of grade 3-4 gastrointestinal/genitourinary toxicity in 117 patients treated with template-based ISBT for recurrent gynecologic malignancies. Kanemoto et al. [47] reported 18% of grade 3 toxicity in 11 patients who received CT-based ISBT ± EBRT for post-surgical vaginal recurrence. Because MRI is more accurate than CT in defining CTV volume (and therefore smaller and more selective treatment volumes), there are logical consequences. In order to reach curative doses in CT-based patients, there are higher doses on the rectum and bladder. This may be the reason for the resulting differences in toxicity between both groups (significant in the case of rectal).
Special mention refers to the 10 patients who received re-irradiation. The interval between irradiations was very heterogeneous among the patients. All received exclusive BT, with a median total prescription dose similar to non-reirradiations. Toxicity was not higher in this population. Among re-irradiated patients, only one treated with MUPIT reported grade 2 proctitis (a rectal ulcer) and urethral stenosis. This patient previously received 45 Gy EBRT and 40 Gy LDR-BT, with 168 months interval between treatments. CTV volume in this patient included the entire length of the vagina, and rectal D2cc was 23.8 Gy, well below the median of the series. The rectal ulcer occurred post-biopsy. Urethral doses are not typically reported in our patients.
Conclusions
MRI-based planning is superior to CT-based planning in perineal interstitial brachytherapy (P-ISBT). It enables better definition of CTV, resulting in smaller and more selective treatment volumes. Our results indicate a tendency towards higher D90% CTV doses and lower rectal/bladder D2cc doses, leading to fewer events of late rectal toxicity.



