Purpose
Soft tissue sarcomas (STS) exhibit aggressive biological behaviors and an elevated risk of local recurrence following surgery. The application of radiation therapy (RT) administered pre- or post-surgery, significantly improves local control (LC) rates after procedures, such as local resection or limb-sparing surgery, mitigating the need for amputation in cases of limb sarcoma [1, 2].
Given their relative radio-resistance, soft tissue sarcomas necessitate higher radiation doses for effective local control. However, achieving this dosage may pose challenges with external beam radiation alone.
Brachytherapy (BT) accurately delivers a concentrated high-dose of radiation to the target tissue, preserving nearby normal tissues with precision. This technique enables dose escalation without the risk of normal tissue toxicity. Several studies have indicated an increased LC rate when adjuvant post-operative BT was applied, either with or without external beam radiation therapy (EBRT) [3-6].
Various research groups, including novel efforts of the Memorial Sloan-Kettering Cancer Center team, have explored the role of local brachytherapy as a supplementary treatment to radical surgery [7-9]. In particular, intra-operative high-dose-rate (HDR) brachytherapy, following wide local excision, has demonstrated a significant enhancement in local control of STS compared with surgery alone [10, 11].
Our treatment protocol involved patients who were treated with perioperative high-dose-rate brachytherapy (PHDRB) with or without post-operative EBRT. In this manuscript, we described the detailed technique of perioperative interstitial brachytherapy as well as our observations and experiences using this modality in soft tissue sarcomas of extremities.
Material and methods
Between October 2020 and October 2023, seven patients with soft tissue sarcomas (4 males and 3 females) were treated with conservative surgery and PHDRB. PHDRB dose ranged from 3.0 to 3.5 Gy BID (inter-fraction interval of at least 6 hours), in 8-10 fractions (total dose of 24-30 Gy). EBRT was delivered in 4-5 weeks after surgical procedure (45-50 Gy in 25 treatments, 1.8-2.0 Gy daily fractions). Higher range of radiation dose was used only in 3 patients with large tumors (13 cm), high-grade tumors, and positive tumor margins, where local control is critically important. Dose was escalated to meet the need to achieve a radiobiological equivalent dose for aggressive disease, balancing the risk of recurrence against the risk of toxicity. Additionally, in these instances, high-risk structures, such as the neurovascular bundle and underlying bone, were intentionally displaced away from catheters using gel foams to minimize toxicity. Dose escalation was only performed in cases where the tumor bed was sufficiently distant from the overlying incision site.
However, patients with smaller tumors (less than or equal to 5 cm and negative margins) were treated with brachytherapy alone. Therefore, the total EQD2 dose delivered with brachytherapy and EBRT was as follow: PHDRB = 24 Gy (regular dose) and 30 Gy (high-dose); EBRT = 50 Gy/25 fractions/5 weeks with 2 Gy per fraction; EQD2 for tissues with α/β10 = 76 Gy; BED = 91.2 Gy (regular dose) and EQD2 = 82.5 Gy; BED = 99 (high-dose); EQD2 for tissues with α/β3 = 78.8 Gy; BED = 131.3 (regular dose) and EQD2 = 86 Gy; BED = 143.3 (high-dose). Surgical and radiation oncology teams were cooperating, utilizing pre-operative physical and imaging data, surgical findings, and gross examination of surgical specimen to collectively identify the implantation site. Imaging included MRI of the involved extremity and contrast-enhanced computed tomography (CECT) of the chest to exclude any lung metastatic disease. Typically, the entire surgical bed was selected for implantation. Pre-operative surface marking of the tumor, along with a 1 cm margin, was carried out.
Brachytherapy technique
Plastic catheters, positioned as closely parallel as possible, covered clinical target volume (CTV) at 1.0-1.5 cm intervals, with a 1 cm margin in all directions. A uniform single-plane implant array was employed for all patients, with catheters inserted perpendicular to the incision site. Additional catheters extending beyond CTV were used to treat longitudinal margins, while lateral margins were addressed by placing the medial and lateral ends of catheters 1.5-2.0 cm beyond the skin incision. The entrance and exit sites of catheters were placed away from the skin incision to avoid radiation toxicity to the skin. In instances, where the bone and neurovascular bundles were in proximity to the tumor bed, gel foam or muscle flaps (not thicker than 5 mm) were introduced between the catheter plane and radiosensitive structures. Subsequently, catheters were secured at the skin exit points using plastic buttons and silk sutures. Following the completion of implantation, surgical team proceeded with the closure. Patient characteristics are shown in Table 1.
Table 1
Patient characteristics
3D dosimetry guidelines
A CT scan for verification was conducted on the third day after surgery, allowing sufficient time for post-operative edema to subside, and ensuring the patient was in a stable condition for transportation. CT examination was then transferred to radiation treatment planning system for dosimetry. The treatment planning process adhered to Paris system, incorporating manual optimization for each of CT slices. Additional guidelines stipulated that clinical target volume (CTV) should be enclosed by a 100% isodose line, defined as the minimum tumor dose (MTD) isodose line in accordance with the ICRU No. 58 recommendations [12]. Planning and normalization were done at the basal points, which were taken at 5 mm to 8 mm from each catheter. Dose homogeneity index (DHI) of 0.5-0.7 was accepted as well as CTV100 of more than 80% and CTV90 of more than 90%. Care was taken to avoid the overlap of 200% isodose lines. MTD to mean central dose (MCD) ICRU No. 58 ratio of not less than 70% was accepted, provided that the volume encompassed by 6 Gy isodose (V150) was not greater than 50% of the volume encompassed by 4 Gy isodose (V100).
Upon approval of the treatment plan, it was transmitted to an afterloader unit control console for thorough verification of dwell positions and treatment times by radiation technician. Brachytherapy delivery commenced on the fifth day post-surgery. Additional brachytherapy parameters are presented in Table 2.
Table 2
Brachytherapy parameters
The number of catheters inserted varied from 9 to 15, depending on the tumor size, with a 2 cm margin in the cranio-caudal extent to ensure comprehensive coverage of the operative bed. The median volume receiving 100% of the prescribed dose was 110.7 cc. The complete procedure with catheter placement into tumor bed and planning images are illustrated in Figures 1-6.
Fig. 1
A) Positioning of catheters at the tumor bed intra-operatively. B) After wound closure. C) Two weeks after removal of catheters
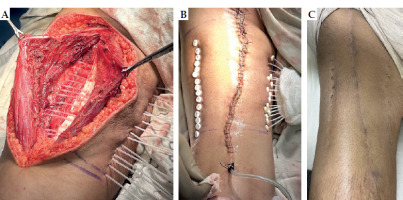
Fig. 2
A) Pre-operative MRI picture of malignant peripheral nerve sheath tumor in the upper thigh. B) X-ray of post-op surgical clips at the tumor bed showing cranial, caudal, and posterior extent
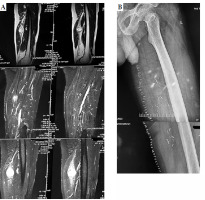
Fig. 3
Isodose distribution around catheters of interstitial brachytherapy at the tumor bed. A) Coronal section of posterior aspect of the thigh. B) Posterior aspect of the thigh and upper third of the leg. C) Anterior aspect of the thigh. D) Axial section
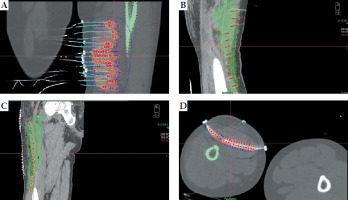
Fig. 4
Catheter placement and isodose distribution in axial, coronal, and sagittal planes, and volume-based distribution in three patients. A) Case 1, B) Case 2, C) Case 3
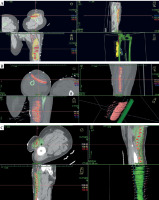
Fig. 5
Dose-volume histogram (DVH) of a patient treated with perioperative high-dose-rate brachytherapy (PHDRB)
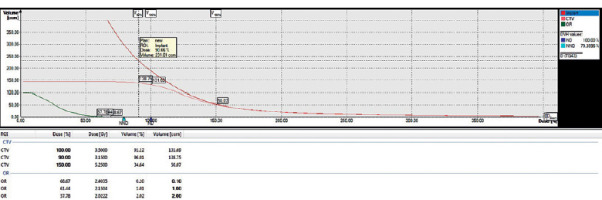
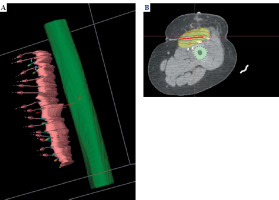
Fig. 6
A, B) Photographs of isodose distribution in the tumor bed and catheter placement with respect to the underlying bone (femur)
All seven patients tolerated their treatments well, and did not experience either acute or late radiation-related complications. Although there was slight serous discharge upon removal of the plastic catheters at the end of brachytherapy treatment, no signs or symptoms of active infection were observed, and post-operative wound healed smoothly. No additional pain management was required during brachytherapy, apart from regular post-operative pain administration.
Following treatment protocol, patients underwent adjuvant chemotherapy after 3 weeks based on specific indications, and were subjected to regular follow-up. Follow-up included a thorough clinical examination, with particular attention to the local site. An MRI of the extremity was recommended if clinically indicated. Additionally, if any symptoms suggestive of lung metastasis were observed, a PET-CT was advised if affordable; otherwise, CECT of the chest was performed. Despite successful local disease control in all seven patients and no recurrences at local disease sites, three patients developed distant metastases. Lung metastases were observed in all three cases, with histology revealing undifferentiated pleomorphic sarcoma in two patients and malignant peripheral nerve sheath tumors in one case. In one patient, lung metastasis was detected on a PET scan three months after treatment completion, while in the other two cases, lung metastases were found one year after treatment completion. Unfortunately, one patient succumbed after 39 months, and another after 16 months, both due to complications of metastatic disease in the lungs. Treatment outcomes are outlined in Table 3.
Table 3
Treatment outcomes
Case 1
A 33-year-old female presented with a large swelling of the right thigh measuring 20 × 15 × 12 cm. Biopsy proved to be monophasic synovial sarcoma. Due to unresectable disease, the patient received neoadjuvant chemotherapy (NACT). After four courses of NACT, there was around 40% reduction in the tumor size. The patient underwent tumor resection along with intra-operative interstitial catheter placement into the tumor bed. To avoid radiation overdose to the underlying exposed bone, gel foam was applied to the tumor bed, thus, maintaining a distance of up to 8 mm between the catheters and bone. PHDR brachytherapy was started after fifth post-operative day. A dose of 30 Gy in 10 fractions was delivered with twice-daily fractions. EBRT of 50 Gy/25 fractions/5 weeks was delivered after 3 weeks of PHDRB completion. The patient attends regular follow-ups, with no evidence of local or distant disease after 1 year of the procedure.
Case 2
A 33-year male with MRI-proven mass arising from left sciatic nerve, measuring 3.4 × 5 × 4.4 cm. Biopsy of the mass was suggestive of a high-grade malignant peripheral nerve sheath tumor (MPNST). Immunohistochemistry (IHC) was positive for vimentin S100, with Ki-67 60-70%, and PET suggested localized disease. Tumor resection was performed by PHDRB, with 15 catheters placed into the tumor bed, 1 cm apart. Adjuvant radiotherapy was planned with brachytherapy alone that was started on day 5. 35 Gy in 11 fractions were delivered in 5 days with twice daily fractions, each fraction more than 6 hours apart. Healing was smooth and without any complications. Subsequently, the patient was monitored during follow-up. Three months after treatment completion, the patient developed dry cough and chest pain; therefore, PET-CT was done, revealing FDG avid bilateral lung nodules suggestive of metastasis. Presently, the patient is receiving AIM-based chemotherapy.
Case 3
A 36-year-old female presented with a mass on her left thigh, measuring 8 × 8 × 15 cm. Wide resection through PHDRB with 15 catheter insertions was performed. Radiotherapy doses were administered as shown in Table 1. Detailed histopathology (HPE) revealed alveolar soft part sarcoma with negative resection margins. IHC was positive for MyoD1, CD34, vimentin, S100, desmin, synaptophysin, myogenin, and EMA. She developed lung metastasis after 42 months without any local disease. Presently, the patient is receiving chemotherapy for lung metastasis.
Case 4
A 61-year-old male with biopsy-proven poorly differentiated pleomorphic sarcoma of the left thigh. IHC picture revealed vimentin-positive, patchy-positive SMA, CK, Desmin, CD34, S100-negative, Ki-67 30-35% high-grade. MRI showed solid cystic intra-muscular mass lesion in the left adductor longus, measuring 10.2 × 8.3 × 13.3 cm (AP × TR × CC). Thorax CT was normal. The patient received neoadjuvant chemotherapy (NACT) with doxorubicin 30 mg day 1-3, cisplatin 40 mg day 1-3 × 3 injection cycles. PHDRB was done with 13 catheter implants, 1.5 cm apart. Treatment with dose of 30 Gy with 10 fractions in 2 weeks, followed by EBRT after 3 weeks with 50 Gy/25 fractions/5 weeks was administered. All resection margins were free. The patient developed pulmonary metastasis after 8 months of treatment completion. Local site was healthy. The patient was on palliative chemotherapy, and died after 16 months due to complications from lung metastasis.
Case 5
A 63-year-old male developed stage IIIB disease on the right thigh, with biopsy-proven undifferentiated pleomorphic sarcoma. The patient was given NACT to reduce the tumor size and after 4 courses of chemotherapy, he was found operable and underwent resection by PHDRB with 12 catheter implants. The prescribed radiotherapy dosage was administered as summarized in Table 1. Local disease remained controlled, but the patient developed lung metastasis after 28 months, and died due to the same after 39 months.
Case 6
A 57-year-old male presented with an 8 × 6 × 11 cm solid firm mass of the upper leg. IHC was positive for desmin, S100, and Ki-67 18-20%, and negative for SMA, CD34, SAT B2, EMA, and SOX 10. Surgery involved PHDRB with 15 catheter implants. Brachytherapy dose: 3.5 Gy/6 fractions/3 days, followed by EBRT with 55 Gy/25 fractions/5 weeks. HPE showed extra-skeletal chondroblastic osteosarcoma with positive deep resection and circumferential soft tissue margins. Tumor infiltrated adipose tissue with vascular invasion. The patient received adjuvant MAP-based chemotherapy for 6 courses, and is presently disease-free.
Case 7
A 45-year-old female with a 19 × 38 × 32 cm mass of the left thigh, underwent excision and PHDRB with 7 catheters at 44 Gy/11 fractions/6 days. HPE revealed undifferentiated pleomorphic sarcoma with clear resection margins. The patient received three courses of adjuvant chemotherapy, and is currently disease-free.
Discussion
In the past, the primary treatment for soft tissue sarcomas was exclusively surgery, and the choice of surgical method depended on the extent of resection. It was observed that local recurrence rates were significantly greater with local surgical excision (30-60%) compared with amputation (5-20%) [12, 13]. In an effort to enhance disease control, the impact of adding radiation therapy (RT) to local surgical excision was assessed. The findings demonstrated comparable disease-free survival (DFS) and overall survival (OS) rates to these achieved with amputation [14]. In a prospective study of 126 patients, Harrison et al. achieved a local control rate of 82% with brachytherapy compared with 67% without brachytherapy (p = 0.049). However, the enhanced local control did not result in either decreased distant metastasis or increased disease-specific survival. This improvement was observed specifically in patients with high-grade histology. The authors speculated that this may be related to tumor biology and kinetics. Since low-grade lesions progress through the cell cycle at a slower rate than high-grade lesions, the 4-6-day dwell time of the implant may be too short to eliminate all cells in a radiosensitive phase of the cell cycle. In this regard, external beam radiation may have an advantage over BT for low-grade tumors [15]. Suit et al. documented the absence of local recurrences in 17 patients with low-grade sarcomas larger than 5 cm, who underwent post-operative external beam radiation [16]. Of our cases, four were high-grade, although local control was achieved in three low-grade tumors also. However, we used the size of tumor criteria as per the ABS guidelines to select EBRT.
Petera et al. applied HDR-BT as a boost in 10 patients, who also received 40-50 Gy EBRT. They administered 6-10 fractions (2.5-3.0 Gy each), totaling by brachytherapy with 18-30 Gy [17]. No delayed wound healing occurred, and two patients experienced late toxicity (subcutaneous fistula, paresthesia). We attempted high-dose brachytherapy in patients with a high-risk of local recurrence. On long-term follow-up, excellent local control was observed with no radiation-related toxicity in these heavily treated patients. Therefore, dose escalation with brachytherapy could be considered for appropriate patients.
Alektiar et al. found no significant increase in wound complications between BT monotherapy and surgery alone in a randomized trial (24% vs. 14%, respectively; p = 0.13) [18]. However, Ormsby et al. from the same institute reported significant wound complication rates of 48% when BT was started within the first five post-operative days. The wound complication rate dropped when BT was started at the earliest 5 days post-treatment (17% BT vs. 15% surgery only; p = 0.9) [19]. Nevertheless, the optimal form and time of adjuvant radiation are unclear.
In a literature review conducted by Neugebauer et al., the authors identified factors associated with a favorable prognosis, including primary tumor, negative margin of resection, wide excision of skin (WES) < 4 cm, and BT after post-operative day 5 [20]. According to their findings, complications appeared to be infrequent, primarily affecting peripheral nerves, less frequently the skin, and very rarely resulting in radiation-induced muscle damage and bone loss. While surgical excision remains the gold standard for treating soft-tissue sarcomas of the extremities, there is still no consensus on the optimal form of adjuvant treatment.
Although there is abundant literature on brachytherapy for cervical cancer, limited information exists regarding techniques and long-term outcomes of interstitial brachytherapy for soft tissue sarcomas. Despite the advancements in external beam radiotherapy methods, allowing for a boost dose to the tumor bed with minimal impact on surrounding organs at risk, the use of brachytherapy, especially for sarcomas, is gradually diminishing. Nevertheless, studies suggest that conformal radiotherapy cannot substitute brachytherapy, and the outcomes achieved with brachytherapy remain superior.
Direct placement of the catheters into the tumor bed is crucial for achieving optimal geometry and consequently, the best dosimetry. This approach minimizes the likelihood of errors and risks to nearby structures. The potential of optimization offered by high-dose-rate (HDR) further aids in reducing the dose to nearby organs at risk. In all patients treated with perioperative brachytherapy, excellent local control was consistently achieved, instilling confidence in considering brachytherapy an essential treatment modality for soft tissue sarcomas where indicated. This article focused on the techniques employed in perioperative brachytherapy and presented long-term results. In the current study, we presented the long-term outcomes of high-dose brachytherapy in patients with high-risk disease, such as large tumors (13 cm), close or positive margins, and high-grade disease. However, more data of this nature could significantly contribute to promote brachytherapy as treatment for such cases.
Although the number of patients treated is relatively small to draw a definitive conclusion, even a modest dataset from multi-centric studies can contribute substantially to shaping conclusive results.
Conclusions
In facilities with limited resources and high patient burden, where conformal radiotherapy is unavailable, brachytherapy remains an excellent modality for boosting the tumor bed or serving as a radical radiotherapy treatment. It provides comparable or superior results, and is less time-consuming. A short interval between radiotherapy and surgery appears to improve outcomes [21].



