Summary
In this study, we found that preprocedural right bundle branch block is a strong predictor for permanent pacemaker implantation (PPMI) after transcatheter aortic valve implantation (TAVI) with balloon-expandable transcatheter heart valve (THV) in bicuspid aortic valve (BAV) population as well as self-expandable devices. Shorter membranous septum is an independent risk factor for PPMI after TAVI with Edwards Sapien XT THV in BAV patients. Type 1 L-R fusion is an important consideration for operators prior to TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is a well-accepted alternative to surgical aortic valve replacement for intermediate- and high-risk patients with severe aortic valve stenosis [1]. Over the past decade, the rate of periprocedural complications has decreased [2, 3] and TAVI is increasingly being performed with a minimalist approach [4] and has become a safe and effective procedure with predictable outcomes. Furthermore, procedural outcomes have improved due to increased operator experience and advances in cardiac imaging, particularly using multislice computed tomography (MSCT). Recently, TAVI has been increasingly used to treat patients with a lower risk profile; this approach is supported by the non-inferiority results from the SURTAVI, PARTNER-II, and NOTION trials [5–7]. In the recently published Partner 3 and EVOLUT low-risk trials, TAVI resulted in similar outcomes versus surgery in a low-risk population with a stenotic tricuspid aortic valve [8, 9].
Bicuspid aortic valve (BAV) is the most common congenital heart disease, affecting 0.5% to 2% of the population and with a male predominance [10]. Although bicuspid valves account for approximately 20% of surgical cases in the elderly (> 80 years) [11], BAV stenosis typically occurs at a younger age than is the case with tricuspid morphology. Thus, most patients with BAV stenosis tend to have an intermediate- or low-risk profile. With the predicted expansion of TAVI into lower-risk patient groups, the proportion of BAV is expected to rise. Patients with BAV were initially excluded from all randomized TAVI trials due to young age and anatomical challenges, i.e. a more oval annulus shape, heavily calcified raphe, leaflet asymmetry, and aortopathy. However, outcomes of TAVI in BAV populations have been reported in several observational studies [12–15]. Paravalvular aortic leak and high permanent pacemaker implantation (PPMI) rates have been the Achilles’ heel for TAVI in BAV especially in younger patients. TAVI-related conduction disturbance requiring new PPMI is an important and the most common complication of TAVI and has been associated in some studies with increased mortality and rehospitalization rates [16]. Although predictors of PPMI have been evaluated in many studies involving tricuspid patients, there are a few data on BAV patients in which balloon-expandable (BE) Edwards Sapien XT transcatheter heart valves (THV) were used.
Aim
The aim of the present single-center study was to evaluate the incidence and predictors of PPMI after TAVI with Edwards Sapien XT THV in BAV stenosis.
Material and methods
Study design and patient population
The datasets of 416 patients who underwent TAVI with Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) THV for symptomatic severe aortic stenosis between November 2012 and January 2018 were collected retrospectively. Sixty-two BAV patients were included with evaluation by MSCT and transesophageal echocardiography (TEE). The decision to proceed with TAVI was made with the consensus of a dedicated heart team including experienced cardiovascular surgeons and interventional cardiologists. Baseline clinical, electrocardiographic (ECG), echocardiographic, and MCST characteristics, and procedural and postprocedural details were collected in the TAVI database. Following the procedure, the decision to perform PPMI was based on Class I or II guideline recommendations as determined by 12-lead ECG and continuous monitoring for 3 days [17]. PPMI was performed in patients who developed high-grade or complete atrioventricular (AV) block during or after the procedure and did not recover within 1 week. At the 1-month and 1-year follow-ups, patients did not require PPMI. Written informed consent for the procedure was obtained from all patients and a locally appointed ethics committee approved the research protocol.
All MSCT scans were performed with a second-generation dual-source CT system (SIEMENS SOMATOM Definition Flash; SIEMENS Healthcare, Erlangen, Germany) prior to TAVI. Images were reconstructed at a 0.75-mm slice thickness with retrospective ECG gating and a standard cardiac filter from early systole (0% of the right-right (R-R) interval) to late diastole (90% of the R-R interval).
Preprocedural MSCT datasets were collected retrospectively from ExtremePacs Client and analyzed with the software OsiriX DICOM Viewer (OsiriX Foundation, Geneva, Switzerland) by an experienced operator who decides on the size of the valve and was blinded regarding PPMI. The usual area and perimeter measurements were used for annular sizing combined in a few patients with bicommissural measurements. Membranous septum (MS) length was determined for the first time in the non-reformatted standard coronal view as described by Hamdan et al. [18]. Bicuspid valve morphology was identified as previously described by Sievers classification [19]. Variables that may have an effect on PPMI after TAVI, i.e. demographic and clinical features such as logistic EuroSCORE II, STS score, NYHA, and preprocedural echocardiography and MSCT data such as annulus-LMCA distance, annulus diameter, bicuspid aortic valve type (type 0, type 1 left-right coronary cusp fusion (L-R), type 1 right-noncoronary cusp fusion (R-N), type 1 left-noncoronary cusp fusion (L-N)), MS length and preprocedural ECG data and perioperative and postoperative clinical features such as an inserted valve diameter (23 mm, 26 mm, 29 mm), mean postprocedural aortic valve gradient, and degree of bioprosthetic valve insufficiency, were examined.
Transfemoral Edwards SAPIEN THVs (Edwards Lifesciences, Irvine, CA, USA) were implanted in all patients. All patients received periprocedural heparin with dose adjustments to maintain an activated clotting time of 250 to 300 s. As a result of the measurements, it was decided which one of the Edwards SAPIEN XT THV (23 mm, 26 mm, or 29 mm) would be implanted and they would be implanted so as to allow them to be opened up to 10% more (oversizing) if necessary, according to the measurements. Balloon predilatation was done in all patients. Four patients with AF before TAVI were treated with anticoagulant + clopidogrel for 1 month and then continued anticoagulant after TAVI. In patients without AF, dual antiplatelet therapy was given for 3 months. In 1 patient, only clopidogrel was given before and after the procedure because of Child-B liver disease.
Statistical analysis
Data analysis was performed using SPSS 20.0 for Windows (SPSS Inc, Chicago, IL, USA) and multivariate Firth logistic regression model was obtained using the logistf R package (logistf: Firth’s Bias-Reduced Logistic Regression. R package version 1.24.1. https://CRAN.R-project.org/package=logistf). The Shapiro-Wilk test was used to determine if the variables’ distribution matched the normal distribution. For data with a normal distribution, parametric tests were utilized. For data that did not fit the normal distribution, the nonparametric Mann-Whitney U test was performed. MSCT and echocardiographic results were evaluated primarily by univariate analysis (Fisher’s exact test for categorical variables, t-test for continuous variables, and Mann-Whitney U test for non-normally distributed variables). Associations between categorical variables were assessed with Fisher’s exact test due to low frequencies in the cross-tabulations. P-value < 0.05 was considered statistically significant. Factors that may have an influence on PPMI following TAVI were evaluated in univariate analyses, and variables with a p-value < 0.05 were included in the model for multivariable Firth logistic regression analysis. Then with multivariable Firth logistic regression analysis, it was examined if the variables in the model were independent risk factors with an effect on PPMI. ROC curve analysis was utilized to evaluate the power of MS length to identify patients with PPMI. The Youden index was used to establish the cut-off point. The binomial confidence interval technique was used to obtain the 95% confidence interval of the sensitivity and selectivity values for the cut-off point.
Results
In the study, 58.1% (36 patients) of the patients were male and the mean age was 73.1 ±8.0 years. The patients’ mean STS scores were 5.5 ±1.7 and their mean logistic EuroSCORE II were 12.8 ±5.7. Based on these scores, 93.6% were evaluated as moderate- or high-risk patients. Although 6.4% (4 patients) of the patients were in the low-risk group according to STS and logistic EuroSCORE II, TAVI was chosen by the cardiac team due to additional comorbid features (porcelain aorta, Child-B liver cirrhosis, fragility, and chronic kidney disease). There was no statistically significant difference in baseline characteristics between the groups. The TAVI was carried out successfully in 93.6% of cases. Postoperative and 1-month cardiovascular mortality was 1.6%. Peripheral periprocedural complications of TAVI developed in 3 patients; 2 of them were treated with surgical cut-down using dacron graft and the other patient was treated percutaneously. One patient who underwent percutaneous intervention due to coronary occlusion after TAVI died 3 days later. No new onset AF, stroke and severe paravalvular aortic leak were observed as periprocedural complications. Table I shows the patients’ baseline clinical, demographic, and implanted valve characteristics. Obesity was diagnosed in 13 (20.9%) patients, 36 (58%) patients were overweight, and 13 (20.9%) had normal weight. Bicuspid valve types in the 62 patients included in the study were as follows: 19.4% (12 patients) with type 0 valve, 72.6% (45 patients) with type 1 L-R cusp junction, 4.8% (3 patients) with type 1 R-N junction, and 3.2% (2 patients) with type 1 L-N junction.
Table I
Demographic, clinical, and valve characteristics
[i] CABG – coronary artery bypass graft, COPD – chronic obstructive pulmonary disease, min – minimum, max – maximum, NYHA – New York Heart Association, PPM – permanent pacemaker, STS score – Society of Thoracic Surgeons score, TAVI – transcatheter aortic valve implantation. Data are presented as a number and percentage or mean (SD).
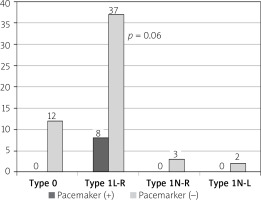
In our study, the incidence of PPMI was 12.9% (8 patients) in the 62 patients with bicuspid aortic valves who underwent TAVI. The distribution of PPMI indications in the patients was as follows: high-degree AV block in 4 (6.4%) patients, ventricular asystole in 2 (3.2%) patients, and left bundle branch block (LBBB) in 2 (3.2%) patients with prolonged PR interval. It was determined that all patients with PPMs were those with type 1 L-R fusion (p = 0.06) (Figure 1). The patients’ median ejection fraction was 50% (min.–max., 24–60), mean transthoracic echocardiogram (TTE) annulus diameter was 2.31 ±0.2 cm, mean MSCT annulus diameter was 25.5 ±2.1 mm, median annulus-LMCA distance measured by MSCT was 13.5 (min.–max. 10–22) mm, and aortic valve area median was 0.68 (min.–max. 0.32–0.94) cm². The MS length median value measured by MSCT was 8.4 mm (min.–max. 4.14–12.7 mm), and MS length was significantly shorter in the univariate analysis in patients who underwent PPMI after TAVI (p < 0.0001). Table II shows the patients’ echocardiographic and MSCT data. All 8 patients with PPMs had type 1 L-R patients with bicuspid valve type. Four of the 8 patients with PPMs had 29 mm Edwards SAPIEN XT THV, 3 had 26 mm THV, and 1 had a 23 mm THV.
Table II
Echocardiographic and computed tomography data
Figure 1
All the PPMI after TAVI shown in type 1 (L-R) patients. L-R: left-right, N-R: non-coronaryright, N-L: non-coronary-left. P-value fot type 1L-R vs. non-type 1L-R (type 0 + type 1N-R + type 1N-L)
In the univariate analysis of the 58 patients who underwent MSCT, when the 4 patients who could not undergo CT scans due to chronic kidney disease were excluded, a significant inverse relationship was found between MS length and PPMI (p < 0.001). When the tomography data of the patients who underwent MSCT were examined, the aortic annulus-LMCA distance was found to be significantly longer in patients with PPMs compared to patients without PPMs in the univariate analysis (p = 0.02). When the univariate analyses were assessed to evaluate the electrocardiographic predictors of PPMI across the patient groups, it was seen that the existence of preprocedural RBBB was statistically substantially greater in the PPM group (p < 0.0001) (Table III).
Table III
Electrocardiographic findings
| Parameter | PPM not implanted (n = 54) | PPM implanted (n = 8) | P-value |
|---|---|---|---|
| PR interval [ms]* median (interquartile range) | 160 (160–160) | 170 (160–200) | 0.38 |
| Preprocedural QRS duration [ms] median (interquartile range) | 60 (40–80) | 100 (40–120) | 0.13 |
| Preprocedural RBBB, n (%) | 1 (16.7) | 5 (83.3) | < 0.0001 |
| Preprocedural LBBB, n (%) | 17 (81) | 4 (19) | 0.42 |
| Preprocedural LAHB, n (%) | 11 (100) | 0 (0) | 0.33 |
| Preprocedural first degree AV block*, n (%) | 8 (80) | 2 (20) | 0.60 |
Univariable analysis of the independent risk factors causing PPMI after TAVI due to bicuspid aortic stenosis had a p-value of ≤ 0.05; presence of preprocedural RBBB, shorter MS length and annulus-LMCA distance in MSCT were included in the model for multivariable analysis. Then multivariable Firth regression analysis was used to examine whether the variables in the model were independent risk factors for PPMI (Table IV). Preprocedural RBBB was found to be a strong predictor for PPMI (95% CI: 3.71–653) (p = 0.001). Shortness of MS length was also found to be a predictor for PPMI. A reduction of 1 unit in septum length was shown to increase the likelihood of PPMI by 2.40 times (95% CI: 1.60–4.83) (p = 0.004) (Table IV).
Table IV
Evaluation of effective independent risk factors for PPM implantation after TAVI by multivariable Firth logistic regression analysis
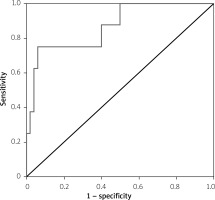
The area under the curve was calculated as 0.867 ±0.071 (p = 0.001) in the ROC curve analysis performed to determine the power of the MS length to discriminate between the patients to be implanted with PPMs (Figure 2). The cut-off point calculated with the Youden index was ≤ 5.79 mm. The sensitivity for this cut-off point was 0.750 (95% CI: 0.349–0.968) and the specificity was 0.940 (95% CI: 0.835–0.988).
Discussion
In the present study we showed that PPMI incidence was 12.9% in patients with BAV undergoing TAVI, all of the patients who underwent PPMI were patients with type 1 L-R fusion, the presence of preprocedural RBBB was a strong factor in predicting PPM after TAVI, and short MS length increased the risk of PPMI by 2.40 times.
To date, there have been limited studies on predictors of PPMI in BAV patients. Eight of the 62 patients in our patient population underwent PPMI after TAVI, making an incidence of 12.9%. The incidence of PPMI after implantation of early-generation THV in patients with bicuspid valves was 14.7% in a meta-analysis [12]. The incidence of PPMI was 16.7% in a series of 48 patients with bicuspid aortic stenosis which underwent TAVI with Edwards SAPIEN XT THV [11]. The incidence of PPM was 14.3% to 40% in a meta-analysis of 8 studies of patients who underwent TAVI for bicuspid aortic stenosis [20]. In a recent single-center study, the incidence of PPMI was 34% in the bicuspid patient group with the novel BE Myval THV system [21]. However, this result is a higher incidence than expected and can be explained by the operators’ first experience with the BE THV system. Recent data showed that a novel BE THV (e.g. Myval) is associated with less conduction disturbances as compared to Sapien 3 THV system in patients with tricuspid aortic valve stenosis [22]. The skirt structure and longer structure of the Sapien 3 THV system than Myval THV system may have contributed to this result. The higher rates of PPMI after TAVI is a well-established fact in bicuspid patients. This result needs to be evaluated in the BAV patients with Sapien XT THV. The low incidence of PPMI and mortality in our study might be explained by the high level of operator expertise, exceeding the learning curve and detailed valve diameter measurement prior to the operation, as well as the fact that oversizing was not more than 10%. New-onset AF and stroke after TAVI are well-known periprocedural complications that affect hospitalization and mortality [23, 24]. In this study, no stroke or new-onset AF were observed in the 1-month period after the TAVI procedure. Moreover, recent data have shown that an increased body mass index is independently associated with the survival benefit after TAVI [25]. In our study, the fact that the majority of patients were overweight (58%) and obese (20.9%) may have contributed to the low mortality with the low periprocedural complications.
The patient population’s bicuspid valve type distribution was as follows: type 0 19.4%, type 1 L-R 72.4%, type 1 R-N 4.8%, and type 1 L-N 3.2%. This distribution was in line with the most important research in the literature that assessed the bicuspid distribution in surgical series [19]. In our study, all of the patients who underwent PPMI were patients with type 1 L-R fusion and this result was very close to statistical significance (p = 0.06). The reason why our findings did not reach statistical significance was the relatively small number of patients included. In a recent evaluation of a TAVI-directed BAV morphological classification, a possible relationship between PPMI and orientation of leaflet fusion was suggested [24, 26]. In recent studies, an elevated incidence of PPMI in individuals who have tricuspid morphology with significant noncoronary cusp calcification has been demonstrated [27–29]. The underlying reason for this situation is the fact that the noncoronary cusp is adjacent to the AV conduction system [30]. The anatomical proximity of the noncoronary cusp to the AV node and the bundle of His is well known and a calcified noncoronary cusp may cause severe aortic stenosis in patients with bicuspid type 1 L-R fusion, and mechanical compression on the conduction system may be the underlying mechanism for PPMI. Larger investigations in patients with BAV morphology are required to examine this process.
Many publications on tricuspid patients have revealed that the presence of preprocedural RBBB is an independent risk factor for PPMI after TAVI [16, 24, 28, 31, 32]. In our study, RBBB was found in 5 (62.5%) of the 8 patients who had PPMs, which was consistent with the literature, and in univariate and multivariable Firth logistic regression analysis the presence of preprocedural RBBB was a strong factor in predicting PPMI after TAVI, as well for the bicuspid patient group. The existence of preprocedural LBBB was not shown to be related to PPMI in patients with BAV morphology in our research. In a meta-analysis of Edwards SAPIEN XT THV, it was shown that preprocedural LBBB was not related to PPM in the tricuspid patient group after TAVI [33]. Furthermore, in another meta-analysis conducted without device separation, it was found that preprocedural LBBB was not related to PPMI in the tricuspid patient group [32]. In our study, preprocedural LBBB, left anterior hemiblock, first-degree AV block, and preprocedural QRS duration did not increase the risk of PPMI in the bicuspid patient group. This result is consistent with the findings of the PARTNER trial [16]. There are publications in the literature showing that a QRS duration of more than 128 ms before the procedure increases the incidence of PPMI [32]. In this study group the maximum QRS duration was 120 ms. The electrocardiographic data indicated that the presence of preprocedural RBBB in patients with bicuspid valves was a strong predictor for PPMI after TAVI as in patients with tricuspid valves. A study with a larger patient population is required to determine whether preprocedural QRS duration, PR interval, and first-degree AV block are predictors for PPMI after TAVI with Edwards SAPIEN XT THVs in patients with bicuspid valves.
The annulus diameter evaluated by TTE in patients with PPMI after TAVI was not statistically significant in our study, and it was observed that there may be a trend to undergo PPMI as the annulus grows. In TAVI, the diameter of the implanted THV increases as a natural consequence of the increase in the aortic annulus. Maeno et al. showed that the risk of PPMI increases in univariate analysis as the diameter of the implanted THV increases [28]. Similarly, the incidence of PPMI has been shown to increase in the bicuspid patient group as a result of the need for intermediate and extra size Myval THV system [21]. In our study, 4 patients had a 29 mm valve implanted, 3 a 26 mm valve, and 1 a 23 mm valve. Although these results appear to be similar to those of our study, the reason why our findings did not reach statistical significance was the relatively small number of patients included.
It is well known that the MS is adjacent to the bundle of His, which is a part of the conduction system [34, 35]. In our study, when MS length measured in MSCT was evaluated in univariate analysis, it was found to be significantly shorter in patients who had undergone PPMI. In our study, the cut-off point calculated with the Youden index was ≤ 5.79 mm. The short MS shows that it is a strong predictor for PPMI after TAVI in patients with bicuspid morphology, as in earlier studies [18, 24, 28, 36]. Recent data showed that shorter MS length is an independent risk factor for PPMI with Sapien 3 and Evolut-THV systems, except for the ACURATE-THV in patients with tricuspid aortic valve stenosis [37]. This result limits MS shortness to be considered a generalizable risk factor for all THVs. Therefore, this study is important in showing that short MS is an independent PPMI risk factor for Edwards Sapien XT THV in bicuspid patients. In our study, the distance between the aortic annulus and the left main coronary artery measured by MSCT was significantly longer in the group with PPMs in the univariate analysis. However, in contrast to our study, it has been shown that the frequency of new LBBB increased with shortening of the annulus-LMCA distance in the tricuspid patient group [38]. This result may have been caused by the tendency to implant the valve deeper to avoid coronary obstruction in patients with short annulus-LMCA distance. In a large meta-analysis evaluating the frequency of aortopathies in bicuspid valve types recently in the literature, 10,021 bicuspid patients were evaluated, and sinus valsalva dilatation was found to be significantly larger in patients with bicuspid valve type 1 L-R fusion compared to other groups [39]. The fact that all patients with PPMI in our study were type 1 L-R may have caused the annulus-LMCA distance to be statistically significant in the univariable analysis. This result may be clinically valuable for the bicuspid patient group. Therefore, studies with a larger bicuspid patient group are needed to evaluate whether the annulus-LMCA distance affects the risk of PPMI in patients with BAV undergoing TAVI.
Limitations. Some important limitations should be considered regarding our study. The retrospective design means there is a bias in the choice of the BAV group. The study is single-center and retrospective, and generalization of the outcomes may not be applicable. Studies with larger numbers of patients are needed. The study population was derived from an older, higher surgical risk TAVI cohort, limiting its generalizability to other patients with BAV. We mainly used Edwards Sapien XT THV. Oversizing of THV was not measured in our study as it is not routinely done above 10% but it was shown to be an important determinant. Although our center is highly experienced in TAVI, operator experience may differ between centers. In addition, type 1 (L-R) was predominant in our population. Larger studies need to evaluate whether certain morphologies increase the risk of PPMI.
Conclusions
The expanding indications of TAVI require randomized trials to prevent its complications. Ours is among the studies with the lowest PPMI rate in BAV patients. In addition, the present study showed that shorter MS and preexisting preprocedural RBBB on ECG are independent risk factors for PPMI in BAV morphology after TAVI with a BE Edwards Sapien XT THV device as the self-expandable device. It is a remarkable result that all patients who underwent PPMI had type 1 L-R morphology. Our results should be supported by larger prospective studies with large participation and newer BE devices in BAV patients.