Purpose
Cervical cancer is the fourth most common cancer among females globally, with approximately 662,301 new cases reported annually (GLOBOCAN 2022) [1]. Despite a declining trend worldwide, it remains a significant cause of morbidity and mortality, especially in low- and middle-income countries (LMICs) [2].
Advancements in treatment modalities over the past two decades have improved outcomes in cervical cancer, including enhanced survival rates and reduced toxicities. Radiation therapy (RT) has evolved from two-dimensional (2D) to three-dimensional (3D), encompassing external beam radiation therapy (EBRT) and brachytherapy (BT) [3]. Image-based adaptive BT, incorporating volume-based target delineation and dose prescription, aims to tailor treatment to individual tumor responses and minimize radiation doses to organs at risk (OARs), thereby reducing toxicities [4].
Traditionally, RT planning has considered doses to OARs, such as the rectum and urinary bladder [5]. Recently, the vagina has emerged as a critical OAR, with vaginal toxicities potentially impacting patients’ quality of life, particularly sexual function. Recto-vaginal point (ICRU rectal point) has been investigated as a dose-limiting point for vaginal toxicities [6]. The concept of posterior-inferior border of symphysis (PIBS) points has been introduced to define dose constraints for the vagina and mitigate toxicities to this organ [7].
The incidence of vaginal toxicities following definitive chemoradiation in cases of intact cervical cancer has been sparingly reported in the literature, with varied results. Therefore, the aim of the current systematic review was to address this knowledge gap, providing researchers with insights into radiation-induced toxicities in this vital organ for future studies.
Material and methods
Our review protocol was registered with Prospero, an international prospective register of systematic reviews (CRD42023396673) [8], and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist 2020 guided our protocol development and review conduct [9, 10]. Institutional ethical review was not required, as patients’ identifiers were not disclosed. The primary objective was to estimate the incidence of vaginal toxicities following radical concomitant chemoradiation, with the end-point being the percentage of patients reporting any form or grade of vaginal toxicity. Secondary objectives included estimating the radiation tolerance dose to the vagina, and exploring associations between vaginal toxicities and other factors observed in individual studies.
Study selection
All studies, except for reviews, book chapters, systematic reviews, and meta-analyses, conducted on intact uterine cervical cancer patients post-radical chemoradiation reporting vaginal toxicities were included. Only English language full-text publications till the date of review were included.
Studies on post-operative cases or those undergoing surgery at any time during management were excluded. Also, studies omitting intra-cavitary radiation therapy (ICRT), or with transperineal implant/template brachytherapy were excluded. Other exclusion criteria were use of non-platinum chemotherapy agents or prior pelvic radiotherapy.
Search methodology
PubMed, Google Scholar, and Cochrane databases search using the following key words: “Vaginal Toxicity”, “Cancer Cervix”, “Radiotherapy”, “Vagina”, and “Tolerance”, was conducted. The searched references were imported to Covidence software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia, available at www.covidence.org) using Zotero reference manager. Careful screening by reading the title and abstract was done by two reviewers independently. Full texts were retrieved, and any unavailable texts were manually searched through journal websites or free retrieval sources. In studies with incomplete reporting of vaginal toxicities or RT doses to the organ, the corresponding authors were contacted via e-mail for additional inputs for the review.
Data extraction
Data were independently extracted by two reviewers and synthesized under pre-defined headings, including study characteristics, population, RT treatment details, toxicity criteria, vaginal toxicities, associated factors, and timing of toxicity recording. In case of any discrepancy, there was a discussion to reach a consensus, which was considered final. Data were tabulated and presented descriptively.
Statistical analysis
All statistical analyses were performed using Jamovi Project (version 2.3), with two-tailed p-values ≤ 0.05 considered significant. DerSimonian and Laird random effects models estimated pooled proportions of vaginal toxicities, and forest plots depicted individual and pooled effects of studies. Heterogeneity was assessed using Cochran Q, τ2, and I2 statistics. Publication bias was evaluated with fail-safe N analysis, Kendall’s tau rank correlation test, and regression test, with a funnel plot indicating bias presence.
Results
Study search and characteristics
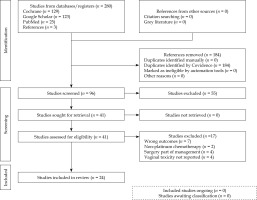
A total of 280 studies were retrieved, of which 24 were included after assessment. Thirteen were retrospective, eight prospective, two randomized controlled trials, and one qualitative research. The entire process is depicted in PRISMA flowchart (Figure 1).
Patient characteristics and treatment details
The included studies encompassed stage IB to IVB cervical cancer patients treated with external beam radiation therapy (chemoradiation), followed by intra-cavitary brachytherapy. Table 1 summarizes treatment details.
Table 1
Patient characteristics and treatment details of included studies
| Study | Country | Study design | Population | No. of patients | Vaginal extension | EBRT dose | BT dose/EQD2 |
|---|---|---|---|---|---|---|---|
| Kirchheiner 2014 [16] | Austria | Prospective observational (EMBRACE) | IB to IVB | 588 | Upper 1/3: 236/588 (40%) Lower 2/3: 30/588 (5%) | 45-50 Gy in 1.8-2.0 Gy/fx. | – |
| Kirchheiner 2016 [6] | UK | Prospective (EMBRACE sub-study) | IB to IVB | 630 | Upper 1/3: 260/630 (41%) Middle 1/3: 19/630 (3%) Lower 1/3: 13/630 (2%) | 45 Gy (IQR, 45-46) | Median HR-CTV D90 EQD2 (Gy): 90 (IQR: 86-94) |
| Westerveld 2022 [17] | UK | Retrospective (sub-study of EMBRACE I study) | IB to IVA | 301 | Upper 1/3: 109/301 (36%) Lower 2/3: 17/301 (6%) | 45-50 Gy in 1.67 to 2.0 Gy/fx. with weekly cisplatin of 40 mg/m2 | – |
| Kumar 2016 [11] | India | Prospective randomized | IIB to IIIB | 37 | – | 50.4 Gy in 28 fx. Inj. weekly cisplatin of 40 mg/m2 | EQD2 EBRT and ICRT combined point A: 79.3 Gy HDR arm (7 Gy × 3 fx.), 78.9 Gy PDR arm (27 Gy/1 fx. over 39 hours, 0.7 Gy each) |
| Rai 2014 [21] | India | Prospective | IB2 to IIIB | 35 | Overall: 11/35 (31.4%) | 46 Gy in 23 fx. weekly cisplatin of 40 mg/m2 | 7 Gy × 4 fx. MRI-guided image-based |
| Tharavichitkul 2014 [22] | Thailand | Prospective cohort | IIB to IIIB | 26 | – | 45 Gy | – |
| Murakami 2021 [13] | Japan | Retrospective | IB2 to IVA | 469 | – | – | Median HR-CTV D90 EQD2 (Gy): 66.1 (51-102) low-risk, 67.5 (41.3-97.3) (others) |
| Kaidar- Person 2014 [23] | Israel | Retrospective | IB1 to IIB | 50 | – | 39.6-50.4 Gy | – |
| Gondi 2012 [12] | United States | Retrospective | IB1 to IVA | 374 (total) 179 (CRT arm) | – | 39.6-50.4 Gy | – |
| Okonogi 2022 [24] | Japan | Retrospective | IB3 to IVA | 36 | – | 45-50 Gy | Mean HR-CTV D90 EQD2 (Gy): 74.7 ±9.4 Gy (3D-IGBT) and 74.8 ±7.6 Gy (HBT) |
| Susko 2016 [14] | United States | Retrospective | IB to IIIB | 62 | – | – | – |
| Dankulchai 2022 [25] | Thailand | Retrospective cohort | IB2 to IVA | 97 | Overall: 81 (83.5%) Arm I: 59 (60.8%) Arm II: 22 (22.7%) | – | – |
| Fidarova 2010 [26] | UK | Retrospective | IB to IV | 34 | – | 45.0-50.4 Gy | 7 Gy × 4 fx. |
| Conway 2020 [27] | Canada | Qualitative research | IB to IVA | 67 | – | Median: 45 Gy | Mean: 28 Gy Mean HR-CTV D90 EQD2 (Gy): 92 ±7 |
| Tse 2016 [28] | UK | Retrospective | – | 100 | – | 45.0-50.4 Gy in 25-28 fx. | Mean HR-CTV D90 EQD2: 88.2 |
| AtaseverAkkas 2021 [29] | Turkey | Retrospective | IA to IIIC2 (FIGO 2018) | 50 | Upper 1/3: 32 (64%) Arm I: 14 (28%) Arm II: 18 (36%) Lower 2/3: 3 (6%) Arm I: 2 (4%) Arm II: 1 (2%) | Median 45 Gy in 25 fx. (45.0-50.4 Gy) | 7 Gy × 4 fx. Mean HR-CTV D90 EQD2 (Gy): Arm I: 85.58 ±7.32 Arm II: 82.73 ±6.73 |
| Tharavichitkul 2021 [30] | Thailand | Retrospective | IB to IV | 180 | Arm I: 47/92 (51.1%) Arm II: 60/88 (68.2%) | 45.0-50.4 Gy | 6-7 Gy × 4 fx. |
| Brand 2006 [15] | Australia | Retrospective | IB to IVA | 179 | Upper 2/3: 17/179 (9.5%) Lower 1/3: 3/179 (1.7%) | Mean: 48.3 Gy | Mean: 23.4 Gy |
| Ruanla 2022 [31] | Thailand | Prospective observational | I to IV | 54 | – | – | 6-7 Gy × 4 fx. |
| Misra 2018 [32] | India | Randomized controlled trial | IB to IVA | 156 CTRT (n = 79) | – | 50 Gy in 25 fx. cisplatin 35 mg/m2 (max, 50 mg) | 6 Gy × 3 fx. |
| Alam 2019 [33] | India | Randomized controlled trial | IIB to IIIB | 72 | – | 50 Gy in 25 fx. | 8 Gy × 3 fx. |
| Sadiq 2020 [34] | Pakistan | Prospective | – | 55 | – | 45 Gy in 25 fx. | 7 Gy × 4 fx. |
| Tharavichitkul 2015 [35] | Thailand | Prospective | IB to IVA | 29 | – | 50 Gy in 25 fx. | 6.5-7.0 × 4 fx. to point A (TAUS-guided) Mean total point A dose: 76 ±10 Gy |
| Saibishkumar 2006 [36] | India | Retrospective | I to IV | 1,069 | – | 35-46 Gy in 15-23 fx. | HDR: 9 Gy × 2 fx. LDR: 35-40 Gy LDR equivalent (1-2 sessions) Median total point A dose: 81 (46-91) Gy |
[i] EMBRACE – image-guided intensity-modulated external beam radiochemotherapy and MRI-based adaptive brachytherapy in locally advanced cervical cancer, EBRT – external beam radiotherapy, BT – brachytherapy, EQD2 – equieffective dose at 2 Gy per fraction, HR-CTV D90 – high-risk clinical target volume dose to 90%, ICRT – intra-cavitary radiation therapy, HDR – high-dose-rate, LDR – low-dose-rate
Primary and secondary outcomes
Table 2 illustrates vaginal toxicities, their correlations with radiation doses, and associated factors. Noteworthy was the considerable variability in the incidence of vaginal toxicities across studies, as shown in Figure 2.
– Grade: The mean incidence of grade 3 or higher toxicities was 12.74% (common toxicity criteria of adverse events, CTCAE version 4.0), 0.98% (CTCAE version 3.0), 10.41% (Radiation Therapy Oncology Group [RTOG] and European Organization for Research and Treatment of Cancer [EORTC]-RTOG/EORTC), and 0% (late effects normal tissue task force [LENT]-subjective, objective, management, analytic [LENT-SOMA]).
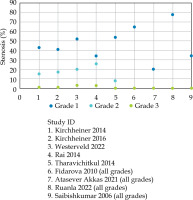
– Type: Vaginal stenosis was the most commonly reported condition, appearing in 9 out of 24 studies, with a median incidence of 61.5% (range, 20-77.8%) (different scoring criteria) (Figure 3). Most studies reported vaginal toxicities without specifying the type. The second most common reported toxicity was telangiectasia/bleeding (Table 2).
Table 2
Primary and secondary outcomes of the included studies in review
[i] CTCAE – common terminology criteria for adverse events, EBRT – external beam radiation therapy, BT – brachytherapy, RV-Ref point – recto-vaginal reference point, IQR – interquartile range, PIBS – posterior inferior border of symphysis pubis, VRL – vaginal reference length, TO – tandem ovoid, TR – tandem ring, HDR – high-dose-rate, PDR – pulsed-dose-rate; VLat 5 mm – vaginal dose at 5 mm lateral distance, D2cc – RT dose to 2 cc volume, D5cc – RT dose to 5 cc volume, D1cc – RT dose to 1 cc volume, D0.1cc – RT dose to 0.1 cc volume, EQD2 – equieffective dose at 2 Gy per fraction
Incidence of vaginal toxicities
Among the studies reviewed, the incidence of vaginal toxicities varied depending on the grading criteria used. Kumar et al. reported only one patient with grade 2 or higher vaginal toxicity out of 37 patients treated with high-dose-rate (HDR) or pulsed-dose-rate (PDR) [11]. Conversely, Gondi et al. observed 30.7% severe late vaginal toxicity (grade 3 or higher, CTCAE v. 4.0) in their study cohort [12]. Murakami et al. reported an 11% incidence of grade 1 or higher vaginal toxicities out of 469 patients [13]. Susko et al. found that 58.06% of patients experienced sub-acute to late toxicities in their retrospective study that included both cervical and uterine cancer cases [14].
The variability in reported incidence can be attributed, in part, to differences in toxicity scoring criteria among studies. Various objective grading systems for vaginal toxicities were employed, including CTCAE (different versions) and institutional criteria. Notably, CTCAE v. 4.0 was the most commonly used toxicity scoring criteria, followed by CTCAE v. 3.0, RTOG/EORTC, and LENT-SOMA. This variation underscores the need for standardization in toxicity scoring methods to facilitate meaningful comparisons across studies.
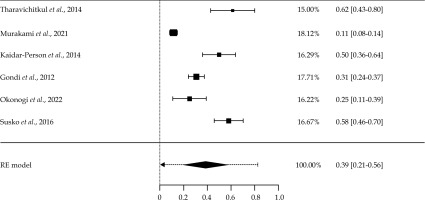
Out of the 24 articles reviewed, only six studies were assessed to estimate the pooled proportion of overall vaginal toxicities. All of these studies used the same toxicity scoring criteria, i.e., CTCAE v. 4. One study (Dankulchai 2022) using the same criteria had to be excluded due to potential data skewness (100% toxicities reported). Of these six studies, five (83%) were retrospective and one (17%) was prospective in nature. Given the significant heterogeneity in study populations and sizes, DerSimonian and Laird random effects (RE) model was utilized for the analysis.
A meta-analysis was conducted to estimate the incidence of overall vaginal toxicities among women. Individual estimates of toxicity proportion in each study and a pooled incidence estimate of toxicity are presented in Figure 4. The overall pooled estimate of vaginal toxicity proportion was 39% (95% confidence interval [CI]: 21-56%). The predictive interval ranged from 8% to 81%. High statistically significant heterogeneity was observed (Tau2 = 0.025, I2 = 95.75%, p < 0.001), indicating heterogeneity in the studies. The reasons for high heterogeneity may include differences in population, sample size, and types of epidemiological studies.
Timing of recording toxicities
The timing of recording vaginal toxicities varied among the studies, with majority focusing on late toxicities. However, there was inconsistency in reporting the specific timing of toxicity assessments. Notably, studies segregated based on reporting criteria also exhibited differences in follow-up intervals and timing of toxicity assessments, contributing to heterogeneity in reported outcomes.
Factors associated with vaginal toxicities
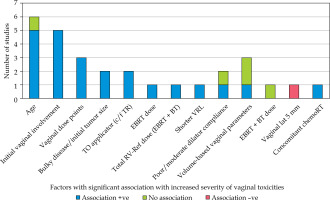
Several factors were identified as potentially influencing the severity and incidence of vaginal toxicities (Figure 5). Age and initial vaginal involvement by disease were found to be significantly associated with increased vaginal toxicities in a subset of studies. Brand et al. noted that vaginal stenosis most commonly developed within the first year post-treatment [15]. Additionally, factors, such as the use of vaginal dilators, concurrent chemotherapy, and specific treatment modalities (e.g., type of brachytherapy applicators) were found to impact vaginal toxicity outcomes in various studies.
Relationship with radiation dose
Five studies included in the review demonstrated a significant correlation between vaginal radiation doses and toxicities (Table 2). Kirchheiner et al. observed a significant association between grade 2 vaginal stenosis and total EBRT and BT RV-Ref dose point in their study cohort [16]. Westerveld et al. similarly found associations between grade 2 vaginal stenosis and various vaginal dose points, with inverse associations observed for certain dose points [17]. Other studies reported associations between vaginal toxicities and point-based vaginal dose parameters, such as D2cc vaginal dose. However, the correlation between vaginal dose volume parameters and toxicity outcomes was not consistent across all the studies (Table 2).
Bias assessment
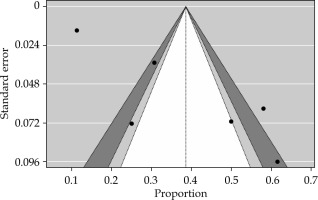
Publication bias assessment indicated minimal bias, with fail-safe N analysis, Kendall’s tau rank correlation test, and regression test, yielding no significant evidence of bias (Figure 6). However, it is essential to acknowledge potential sources of bias beyond publication bias, which could impact study outcome. Risk of bias was found to be low for majority (19/24) of the studies reviewed.
Discussion
Incidence of vaginal toxicities
The meta-analysis revealed a pooled estimate of 39% for the overall incidence of vaginal toxicities (CTCAE v. 4) among cervical cancer patients following definitive chemoradiotherapy. Vaginal stenosis emerged as the most commonly reported toxicity, affecting approximately one-third of all the studies. Notably, severe toxicities (grade 2 or higher) were generally reported at low rates across the studies, regardless of toxicity scoring criteria employed.
The variation in reported incidence rates underscores the importance of standardized toxicity scoring methods to facilitate accurate comparisons and interpretation of study findings. Recently, an Italian survey resulted in wide variation concerning recording and treating vaginal toxicities after chemoradiation, highlighting the need for guidelines in contouring and vaginal RT dose reporting [18]. Additionally, the use of novel approaches, such as the time-weighted adverse event reporting system, may provide valuable insights into temporal trends of toxicities and their impact on quality of life.
Timing of recording toxicities
The lack of uniformity in recording the timing of vaginal toxicities poses a challenge in interpreting study results and comparing outcomes across the studies. While majority of the studies focused on late toxicities, variations in follow-up intervals and timing of toxicity assessments contribute to heterogeneity in reported outcomes. Standardization of reporting protocols for timing of toxicity assessments would enhance the reliability and comparability of study findings. Though not qualified to be included in this review, a recent publication by Chopra et al., a post-hoc analysis of adverse events in PARCER trial on post-operative patients, a newly developed time-weighted adverse event reporting system is worth mentioning here. Instead of a snapshot of worst adverse toxicity grade, MOSES provides temporal trends of toxicity, which gives more valuable inputs to assess quality of life (QoL) [19, 20].
Factors associated with vaginal toxicities
Age and initial vaginal involvement by disease emerged as significant factors associated with increased severity and incidence of vaginal toxicities in several studies. The use of vaginal dilators, concurrent chemotherapy, and specific treatment modalities were also found to influence toxicity outcomes. These findings underscore the importance of considering patient-related and treatment-related factors in assessing and managing vaginal toxicities following chemoradiotherapy.
Relationship with radiation dose
The correlation between vaginal radiation doses and toxicities was evident in multiple studies in this analysis, with various dose points and parameters showing associations with toxicity outcomes. However, inconsistencies in reporting and variability in dose calculation methodologies highlight the need for standardized approaches in dose reporting and evaluation. Further research is warranted to elucidate the optimal dose-volume parameters and treatment strategies to minimize vaginal toxicities while optimizing treatment efficacy.
Limitations
Several limitations should be acknowledged in interpreting the findings of this review. Firstly, the retrospective design of majority of included studies introduces inherent biases and limitations in data collection and analysis. Additionally, the heterogeneity in dose reporting parameters and toxicity scoring criteria across the studies limits the comparability and generalizability of findings. Moreover, the lack of uniformity in recording the timing of toxicity assessments further complicates the interpretation of studies’ results.
Conclusions
In conclusion, this systematic review and meta-analysis provide valuable insights into the incidence and factors associated with vaginal toxicities following definitive chemoradiotherapy in cervical cancer patients. In our analysis, the pooled estimate of incidence of overall vaginal toxicities (CTCAE v. 4) was 39% among cancer cervix cases following definitive chemoradiotherapy. Vaginal stenosis is the most commonly reported toxicity (around one-third of all studies). Severe toxicities (grade 3 or more) are reported to be low in most studies, irrespective of toxicity scoring criteria. Factors, such as age, initial vaginal involvement, vaginal dose points are reported to be associated with vaginal toxicities. Standardization of toxicity scoring methods and dose reporting parameters is essential to facilitate accurate comparisons and interpretation of the studies’ findings. Future research efforts should focus on elucidating optimal treatment strategies to minimize vaginal toxicities while maximizing treatment efficacy and patient outcomes.