Summary
Epicardial adipose tissue (EAT) volume may be related to the 1-year outcome in patients undergoing transcatheter aortic valve implantation for severe aortic stenosis. The EAT volume may serve as a predictor of major adverse cardiac and cerebrovascular endpoints in this patient population. Reduction of the EAT volume may also potentially be a therapeutic target to avoid adverse outcomes in this patient population.
Introduction
Calcific aortic valve stenosis has become the most common valve disease in adults, particularly in developed countries, with an increase in life expectancy and the elderly population [1]. The incidence of aortic stenosis increases with age, affecting approximately 10% of patients over the age of 80 [2]. Aortic valve stenosis is not only associated with aging but also occurs as a result of dynamic inflammatory and oxidative processes [3]. Although various etiological risk factors such as calcification, lipoprotein deposition, and chronic inflammation have been identified for aortic stenosis, it is generally defined as an inflammatory-based disease that shares a common pathophysiology with atherosclerosis [4]. This has encouraged ongoing studies to further investigate the role of inflammation in its etiology. Previously, surgery was the sole treatment option for severe aortic stenosis. However, transcatheter aortic valve implantation (TAVI) has emerged as the preferred approach for patients with intermediate and high surgical risks, and also for those deemed unsuitable for surgical procedures. Inflammatory markers, which play a significant role in the etiology, may serve as predictors of prognostic outcomes in patients undergoing TAVI for severe symptomatic aortic stenosis [5].
Epicardial adipose tissue (EAT), which serves as the primary visceral fat storage of the heart, comprises approximately 20% of the heart’s total weight and covers about 80% of its surface. Its proinflammatory characteristics have captured the attention of researchers and are thought to be linked to various cardiovascular diseases [6]. Physiologically, EAT has several cardioprotective functions. It acts as a physical buffer, protecting the heart from mechanical stress and low temperatures, and serves as an energy reservoir for the myocardium by providing free fatty acids during periods of high metabolic demand. Additionally, EAT produces anti-inflammatory adipokines, which further contribute to its cardioprotective roles [7]. Conversely, EAT exhibits adverse proinflammatory and proatherogenic properties due to its production and release of cytokines such as interleukins, tumor necrosis factor (TNF)-α, and other bioactive molecules [8]. EAT volume has been shown to be associated with cardiovascular disease risk factors, metabolic syndrome, and vascular calcification. Intrathoracic EAT is believed to exert a direct toxic effect on vascular tissues and locally impact the heart and coronary arteries by inducing inflammation through the secretion of autocrine and paracrine cytokines [6]. In addition, EAT thickness has been shown to be associated with left ventricular mass and severe left ventricular remodeling patterns in patients with severe AS [9].
Aim
Therefore, in this study, we aimed to investigate the effects of EAT volume, which has inflammatory effects, on the prognosis of patients who underwent TAVI.
Material and methods
Study design
The medical records of 421 patients who underwent TAVI for symptomatic severe aortic stenosis at the Cardiovascular Department of Bilkent City Hospital (Ankara, Turkey) between March 2018 and December 2022 were reviewed. Patients who underwent TAVI and had pre-procedural computed tomography (CT) examinations were included in this study. Patients with severe anemia (hemoglobin < 10 g/dl), low platelet count (≤ 100,000/dl), chronic renal failure, glomerular filtration rate (GFR) < 30 ml/min/1.73 m2, liver cirrhosis (Child-Pugh class C), active infection or sepsis, active malignancy, and under chemotherapy were excluded from the study. Patients who underwent emergency TAVI, those who received TAVI via non-transfemoral access routes, and those who had valve-in-valve TAVI procedures were excluded from this study.
The decision to perform TAVI was made by the heart team for all patients. Echocardiographic measurements were evaluated by a heart imaging specialist, and the severity of aortic valve stenosis was determined on the basis of the current guidelines before all TAVI procedures [1]. Before all decisions regarding TAVI were made, surgical risk was calculated using the EuroSCORE II risk calculator (EuroSCORE.org/index.php?id=17). The heart team at our hospital consisted of at least two clinical cardiologists, two invasive cardiologists, two cardiovascular surgeons, and anesthesiologists, each recognized as an expert in their respective fields. In some cases, pulmonologists, oncologists, endocrinologists, and/or other specialists were consulted depending on the patient’s condition.
CT protocol
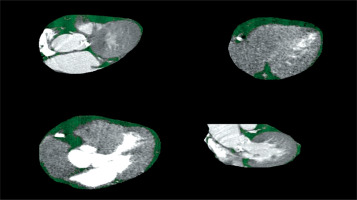
Images were obtained using a 512-slice CT device (GE Revolution 512-Slice Computed Tomography, Waukesha, WI, USA). Initial non-contrast images were acquired, followed by contrast-enhanced images with retrospective gating. The acquisition parameters were as follows: 120 kV; 100 mAs; pitch, 0.9; rotation time, 0.6 s; and 80 mm. For contrast-enhanced imaging, 90 ml of contrast material was administered via an automatic injector at a rate of 5 ml/s from the left antecubital vein while the patient was in the supine position. At the end of the contrast medium administration, 40 ml of 0.9% saline was administered at the same rate. In this study, a semi-automatic method was used to assess epicardial fat volume and analyze the interobserver variability. Coronary CT angiography images of the patients were evaluated using the GE AW Volumeshare 7 workstation. To measure the EAT volume, cardiac contours were drawn manually on axial-plane CT images obtained from the thoracic inlet level to the cardiac apex. Values between –190 HU and –30 HU were used for EAT quantification. Contour correction was performed manually by controlling the epicardial fat contours and the epicardial fat tissue volumes of the patients were recorded (Figure 1). All measurements were performed by two independent, experienced radiologists. If the measurements by the two investigators differed by more than 5% for any variable, the patient was excluded from the study. If the difference was less than 5%, the measurements were averaged.
Endpoints
According to the second Valve Academic Research Consortium (VARC) criteria, major adverse cardiac and cerebrovascular endpoints (MACCE) are defined as all-cause death within 1 month, all-cause death within 12 months, intraprocedural blood transfusion, emergency pacemaker implantation, rehospitalization, the need for pacemakers during follow-up, major bleeding, acute renal failure, major vascular complications, stroke, transient ischemic attack, hemorrhagic stroke, and acute coronary syndrome [10]. One-month mortality was defined as death from any cause in the first month after TAVI, or death if the initial hospital stay was longer than 1 month.
Statistical analysis
SPSS for Windows (ver. 23.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. Parametric variables were presented as mean ± standard deviation; non-parametric variables were presented as median with 25–75th percentile; and categorical variables were assigned as percentages. Continuous variables were analyzed using the Kolmogorov-Smirnov test for normal distribution. Depending on whether the continuous variables were normally distributed, differences between the groups were evaluated using Student’s t-test or the Mann-Whitney U test. The χ2 test was used to compare categorical variables between groups. Univariate and multivariate Cox regression analyses were performed to investigate the predictors of 1-year MACCE in the study population. Variables that were significant at p < 0.05 in the univariate analysis were included in the multivariate logistic regression analysis to identify independent predictors of 1-year MACCE (pacemaker requirement at follow-up, major bleeding, ischemic stroke, hemorrhagic stroke, acute myocardial infarction, all-cause death at 1-month, all-cause death at 1 year). A receiver operating characteristic (ROC) curve was used to determine the cutoff EAT value for predicting MACCE. The Kaplan-Meier survival analysis of patients divided them into two groups based on the EAT cutoff interval, and survival changes were calculated using a log-rank test.
Results
The medical records of 421 patients were reviewed. Forty-five patients with anemia, three with low platelet counts, 23 with chronic renal failure, 5 with non-femoral access, 10 with missing or questionable radiological results, and 1 with chronic liver disease were excluded from the study. These patients were stratified into two groups based on the presence or absence of major adverse cardiovascular and cerebrovascular events (MACCE). According to the VARC criteria, 52 patients were classified into the MACCE group, while 282 patients were assigned to the no-MACCE group.
The study included patients with a mean age of 75.9 ±7.4 years, of whom 46.7% were male. EuroSCORE Q1-Q3 was higher in the MACCE group than in the no-MACCE group (median, 7.5 vs. 5.4, respectively; p = 0.029). Mean systolic pulmonary artery pressure (PAP) was higher in the MACCE group than in the no-MACCE group (46.6 ±15.2 vs. 41.6 ±13.5, respectively; p = 0.038). Mean creatinine level was higher in the MACCE group than in the no-MACCE group (1.06 ±0.33 vs. 0.95 ±0.26, respectively; p = 0.006). Mean albumin level was lower in the MACCE group than in the no-MACCE group (38.3 ±5.6 vs. 40.6 ±5.0, respectively; p = 0.004). Mean EAT volume was higher in the MACCE group than in the no-MACCE group (120.7 ±43.9 vs. 96.1 ±39.8, respectively; p < 0.001). The demographic, clinical, and laboratory characteristics of the patients are shown in Table I.
Table I
Demographic, clinical and laboratory characteristics of patients
[i] AVA – aortic valve area, CABG – coronary artery bypass graft, COPD – chronic obstructive pulmonary disease, CVA – cerebrovascular accident, EAT – epicardial adipose tissue, HDL – high-density lipoprotein, HU – Hounsfield unit, LDL – low-density lipoprotein; LVEF – left ventricular ejection fraction, PAP – pulmonary artery pressure, PCI – percutaneous coronary intervention, WBC – white blood cells.
Following TAVI, 13.5% of patients required emergency pacemaker implantation. At the 1-year follow-up, 2.1% of patients required a pacemaker. Acute renal failure, ischemic stroke, and major bleeding were observed in 2.7%, 2.4%, and 0.06% of the patients, respectively. The 1-month mortality rate was 3.6% and the 1-year all-cause total mortality rate was 6.9%. The procedural and postoperative 1-year follow-up outcomes of the groups are shown in Table II.
Table II
Procedural and postprocedural clinical outcomes of the groups (1-year follow-up)
The results of univariate and multivariate Cox regression analysis for 1-year MACCE are presented in Table III. Univariate Cox proportional-hazard analysis revealed that creatinine and albumin levels, mean systolic PAP, and EAT volume were significantly associated with MACCE. Multivariate Cox proportional-hazard analysis revealed that EAT volume (hazard ratio (HR) = 1.012; 95% confidence interval (CI): 1.006–1.018; p < 0.001) and albumin level (HR = 0.925; 95% CI: 0.866–0.987; p = 0.018) were significantly independently associated with MACCE.
Table III
Univariate and multivariate Cox regression analysis for 1-year MACCE
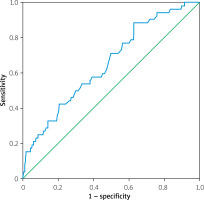
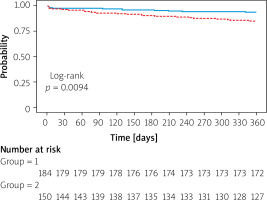
ROC analysis showed that a cutoff value of 103.5 mL for EAT volume predicted post-TAVI MACCE with a sensitivity and specificity of 57.7% and 57.4%, respectively (area under the curve = 0.653; 95% CI: 0.574–0.732; p < 0.001) (Figure 2). The patients were stratified into two groups based on the cutoff interval determined from the ROC curve. The Kaplan-Meier survival plot for patients categorized by EAT volume values is presented in Figure 3. The results showed that the group with low EAT volume developed significantly fewer MACCE over 1 year than the group with high EAT volume (log-rank test: p = 0.0094).
Discussion
This study revealed that EAT volume can be an independent predictor of MACCE, including postprocedural pacemaker requirement, major bleeding, intraprocedural stroke, ischemic stroke, all-cause death at 1 month, and all-cause death at 1 year, in patients undergoing TAVI. We currently lack sufficient data on the impact of epicardial fat tissue on post-TAVI outcomes in patients with severe aortic stenosis. Epicardial fat tissue is significant due to its association with inflammation and its potential as a therapeutic target. The only study on this topic was conducted by Eberhard et al. in 2019, involving 503 patients [11]. This study highlighted that epicardial fat tissue is more prevalent in men and is not correlated with body mass index, but it is associated with both short- and long-term outcomes. Numerous studies have demonstrated the relationship between obesity and EAT volume. Similarly, several reports have shown that TAVI patients with higher BMI tend to have better outcomes. Although the relationship between EAT volume and BMI was not demonstrated in this study due to data limitations, it can be inferred from the literature that there might be a positive correlation between EAT volume and TAVI outcomes.
For many years, aortic valve replacement surgery was the sole treatment option for severe aortic stenosis. However, a significant proportion of patients were ineligible for surgery due to advanced age, comorbidities, and frailty. TAVI has since emerged as an alternative, initially for high surgical risk patients, then for intermediate surgical risk patients, and for those deemed unsuitable for conventional surgery [12]. TAVI is now considered an alternative to surgery even in low-risk patients; however, complications such as in-hospital mortality, valve durability, risk of stroke and bleeding, conduction disorders requiring pacemaker implantation, renal failure, paravalvular leak, and infection remain significant concerns [12]. The mortality rate in the first month after TAVI ranges from 1.8% to 9.0%, and the 1-year all-cause mortality has been reported to be as high as 23.7% in registry studies [13, 14]. In our study, the 1-month all-cause mortality rate was 3.6%, and the 1-year all-cause mortality rate was 6.9%, in accordance with the VARC-2 criteria and after applying the exclusion criteria in this selected patient group.
Epicardial adipose tissue functions as a mechanical barrier by enveloping the heart tissue and may exert adverse effects on the coronary vessels, myocardium, and heart valves due to the secretion of inflammatory cytokines. While numerous studies have investigated the impact of EAT on coronary heart disease, its effects on heart valves have been less extensively studied. In a study of patients aged ≥ 65 years, EAT was associated with CT-detected calcium deposits in the mitral ring and aortic valves [15]. Another study conducted on 225 patients suggested that an EAT thickness ≥ 7 mm may be associated with aortic valve sclerosis [16]. Aortic valve sclerosis is defined as senile calcification and thickening of the aortic valve without stenosis of the ventricular outflow. Unlike this study, in which EAT thickness and aortic valve sclerosis were evaluated by echocardiography, in our study which included patients with severe aortic stenosis, no correlation was found between EAT volume and the Agatston aortic valve calcium score. Another study reported that EAT thickness on echocardiography was significantly higher in patients with severe aortic stenosis than in those without aortic stenosis [17]. In these studies, EAT thickness measured by echocardiography did not accurately reflect the actual amount of EAT due to variations in adipose tissue distribution around the heart. Therefore, true EAT volume should be assessed in three dimensions using cardiac CT or magnetic resonance imaging (MRI) [18].
Cardiac MRI is regarded as the gold standard for imaging EAT. This modality offers superior visualization of both visceral and parietal pericardium, facilitating accurate assessment and volumetric quantification of EAT. Moreover, cardiac MRI does not involve radiation exposure or the use of contrast agents. However, its limitations include restricted accessibility, high cost, unsuitability for patients with claustrophobia, and limited applicability in patients with implanted devices [19]. Cardiac CT is currently the preferred imaging modality for evaluating EAT over other techniques such as echocardiography or cardiac MRI. Due to the distinct attenuation values of fat on CT, EAT can be easily identified on both chest and cardiac CT scans, with or without contrast. Cardiac CT with ECG gating provides precise measurements of EAT volume [18]. While EAT thickness has been commonly utilized in studies investigating EAT and coronary artery disease, EAT volume has been reported to be a more reliable indicator of coronary artery disease [19]. This study is particularly valuable as it demonstrates the prognostic significance of EAT volume without incurring additional costs by utilizing cardiac CT images obtained during TAVI planning, thus establishing this approach as a reliable method for assessing EAT volume.
Few studies have assessed adipose tissue thickness or volume measured by CT in patients undergoing TAVI. In a small-scale study involving 45 patients, Ertas et al. investigated the relationship between EAT thickness, as measured by CT, cardiac conduction disturbances, and outcomes in patients undergoing TAVI. The study did not find a significant relationship between EAT thickness and overall outcomes, likely due to the limited sample size and the low number of endpoints [20]. In a study involving 560 patients, the relationship between EAT volume and pre-existing cardiac conduction abnormalities, as well as the necessity for post-intervention pacemaker implantation in patients with severe aortic stenosis who underwent TAVI, was investigated. The study determined that an EAT volume exceeding 144.3 ml was independently associated with pre-existing first-degree atrioventricular block and the requirement for new pacemaker implantation [21]. In our study, which was conducted with a larger number of patients, 2.1% of the patients required a new pacemaker during follow-up, and EAT volume was found to be significantly related to the need for a new pacemaker during follow-up. Also, an EAT volume > 103.5 ml was significantly associated with the development of MACCE 1 year after TAVI. In our study, the median aortic valve Agatston score was 2851.4, whereas in the previous study, the median valve score was 2509.5. In a study involving 560 patients investigating the relationship between conduction disorders and EAT volume in patients undergoing TAVI, higher EFT volumes are independently associated with pre-existing first-degree AV block and the need for new PPM implantation after TAVI [21].
Epicardial adipose tissue is believed to exert an influence on myocardial tissue, as well as on coronary vessels and valves. In addition to its contribution to valve calcification, EAT thickness has been suggested to affect left ventricular remodeling in patients with aortic stenosis [9]. Left ventricular remodeling is associated with increased cardiovascular morbidity and mortality in patients with aortic stenosis, even if the patient is asymptomatic and the stenosis is not severe [22]. Our study did not include data on left ventricular hypertrophy; however, when evaluating the impact of EAT volume on outcomes, its effect on the myocardium and aortic valve stenosis requires consideration.
The most significant limitation of this study is its retrospective design and the small sample size, with data derived exclusively from the medical records of a single center. The impact of medications used by patients on the outcomes was not analyzed, and only a limited number of patients reached the study endpoint. Additionally, while 1-year follow-up all-cause mortality was assessed, no analysis was conducted regarding the specific causes of death. Furthermore, the use of the EuroSCORE II instead of the STS PROM scale was due to the limitations in the available data. There were also insufficient data on the indications and rates of antiplatelet and antithrombotic treatment, which may have influenced the bleeding-related outcomes observed in the study. Similarly, due to the retrospective nature of the study, frailty was not evaluated, which could have affected the overall risk assessment.
Conclusions
The EAT volume may influence the outcomes of patients undergoing TAVI due to its effects on the aortic valves and myocardium. Larger-scale studies are required to elucidate the impact of EAT on post-TAVI outcomes. Additionally, further research is necessary to evaluate the role of EAT volume in the development of aortic valve stenosis and to determine whether reducing EAT volume could serve as a potential therapeutic target.