Purpose
Breast brachytherapy is a widely used technique that can deliver conformal radiation therapy (RT) in patients undergoing accelerated partial breast irradiation (APBI) and boost, following whole breast irradiation (WBI) [1-4]. Steep dose gradient and isotropic nature of brachytherapy deliver high doses to tumor bed while sparing normal tissue, resulting in excellent cosmetic outcomes and delivering insignificant radiation to the heart and lungs [1, 5-11]. However, recently, the results of FAST-Forward trial suggested that WBI in early breast cancer (EBC) can be safely delivered in a week with comparable oncological outcomes [12]. Throughout years, brachytherapy has been the only modality that can complete RT within five days, with oncological outcomes similar to WBI.
Over the last 50 years, breast cancer’s surgical and adjuvant RT treatment has evolved significantly. Various randomized trials have shown that mastectomy and breast conservation surgery (BCS) with WBI are effective treatment options for EBC, with comparable survival rates [13-18]. However, due to whole breast irradiation’s long- and short-term side effects as well as financial burden associated with daily radiation treatments at a facility for 3-6 weeks, many eligible women choose mastectomy over BCS, especially in India [19-21]. Furthermore, 20% of women undergoing BCS never receive radiotherapy as part of their treatment [22]. More individuals would benefit from BCS if adjuvant RT was safer and more convenient. This could reduce the number of patients treated with BCS who would never get adjuvant RT [23].
Numerous guidelines for breast brachytherapy exist in the West; however, they lack reproducibility for developing countries, such as India. In addition, none of the current patient selection guidelines for APBI can differentiate local control (LC) and primary APBI end-point among the risk groups, whereas the Groupe Européen de Curiethérapie – European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) and American Brachytherapy Society (ABS) guidelines can differentiate disease-free survival (DFS) and overall survival (OS) [24]. Recently published data showed excellent LC and OS in unsuitable, high-risk, and unacceptable patients treated with APBI [25, 26].
This consensus statement of the Indian Brachytherapy Society (IBS) aims to generate practical and reproducible guidelines allowing direct implementation in the Indian scenario as well as stratification of the risk groups.
Material and methods
The IBS board of directors has appointed a panel of physicians with expertise in breast cancer and, in particular, breast brachytherapy, to develop a consensus statement. The project’s goals were to create new and practical guidelines based on a review of new data on the efficacy and toxicity of multi-catheter interstitial brachytherapy (MIB)-based APBI and boost irradiation. First, a literature review addressing clinical outcomes and toxicities associated with brachytherapy by technique was conducted, focusing on randomized trials, prospective studies, multi-institutional series, and single-institution reports. Then, following a discussion on the updated literature, the guidelines were drafted based on authors’ consensus in the Indian context. Under various titles, the recommendations covered general aspects of breast brachytherapy, such as 1) Patient selection, pre-treatment workup, and patient care, 2) Treatment plan, 3) Implant techniques, 4) Target defining, 5) Prescription of dose and dose-rate, 6) Treatment monitoring, 7) Catheter removal, and 8) Post-treatment patient care and follow-up.
Results
Rationale for APBI
In patients treated with BCS without adjuvant RT, the recurrence patterns of breast tumors spurred interest in exploring less-than-whole breast RT. Clinical trial data imply that among the 30% of patients who suffer from recurrences without RT, the vast majority (about 80%) will develop them at an initial tumor site, usually within 2 cm [27-29]. Furthermore, the absolute percentage of recurrences in a region other than the tumor bed is minimal, ranging from 3% to 5%. Therefore, the partial breast irradiation treating only the primary tumor location with a 1-2 cm margin, has a strong rationale supported by clinical, pathological, and radiobiological findings.
Patient selection for APBI
Patient selection is of paramount importance in successfully executing APBI. For example, in a study by Vicini et al., tumor control rates reached outstanding results over a ten-year period when highly selected individuals were treated with a large volume implants, with 2 cm margins of the tumor bed [30]. Budrukkar et al. [24] published long-term outcomes of APBI, where a 1 cm margin from the tumor bed was treated. At a median follow-up of 114 months, 10-year LC, DFS, and OS were 90%, 81%, and 83.5%, respectively, among a 240 women cohort. However, Perera et al. [31] performed implants that solely treated the tumor bed as demarcated by surgical clips, and reported a 5-year recurrence rate of breast tumors as 16%. Furthermore, the recurrence occurred in 2/3 of patients outside the implant volume. These findings imply that the amount of breast irradiation and patient selection criteria would finally influence the success of APBI treatment. Table 1 describes the patient selection criteria advised by IBS, and compares them with other published guidelines.
Table 1
Patient selection criteria
Age: A pathological study by Imamura et al. [32] investigated the distribution of non-invasive disease components in patients with invasive cancer as a function of patient age. In this analysis, the radial distribution of non-invasive cancer was substantially more extensive in patients under the age of 40 compared with older patients. In addition, 65 years old or elder patients with a low- or intermediate-grade non-invasive components were the only categories whose non-invasive component remained within 5 mm of invasive component [32]. Therefore, IBS recommends that APBI can safely be performed in patients above 40 years of age.
Size: Majority of APBI trials, especially with brachy-therapy, did not enroll patients with tumors larger than 3 cm. The presence of invasive foci outside a 2 cm margin from the tumor is independent of tumor size when considering 2-5 cm tumors. The multi-focality is not present in 40% of EBC patients, and only 14-16% of cases have an invasive focal component beyond 2 cm. Therefore, IBS advocates for APBI in tumors up to 3 cm and enrollment into clinical trials for patients with a tumor size between 3.1 and 5 cm. We do not recommend APBI for multi-focal and multi-centric tumors.
Extensive intra-ductal component (EIC): A Milan III study examined the efficacy of an extensive excision (quadrantectomy) with and without WBI. Breast tumor recurrence was observed in 24% in the quadrantectomy-only group vs. 6% in the surgery with WBI group after 10 years. The trial did not examine the effects in specific categories; however, younger patients and those with EIC had a notably high-rates of recurrences [33]. Since, the presence of EIC remains an exclusion criteria in all international guidelines, IBS does not recommend APBI for tumors with EIC.
Hormone status: Few studies analyzed the effect of hormone status, mainly negative estrogen receptor (ER) and progesterone receptor (PR), and positive or negative Her-2/neu, on ipsilateral breast tumor recurrence (IBTR) in patients treated with APBI. Since the results are conflicting, there is no clarity regarding the oncological efficacy of APBI when compared with WBI in non-luminal A type cancers [34-37]. The receipt of anti-Her-2 therapy should also be taken into account, since it can impact the patterns of recurrence. In a single-arm prospective study, Her-2/neu positivity was the only factor in univariate analysis that was associated with higher local recurrence rates [38]. Therefore, it is safer not to consider APBI in Her-2/neu-positive tumor patients. ER and PR status does not seem to affect the selection of APBI patients.
Surgical margins: APBI can be accomplished safely in patients with negative margins, and is contraindicated in cases with positive or close margins. Although several societies accept “no ink on tumor”, IBS strongly recommends a wider margin of 2 mm.
Nodal positivity: NSABP B-39 is one of the most extensive randomized trials, which enrolled for APBI not only lymph node-positive patients [26]. Though not adequately powered, the node-positive sub-group had an hazard ratio (HR) of 1.9 when comparing APBI and WBI (p-value not significant). We currently do not recommend the use of APBI in node-positive EBC patients.
Lymph-vascular space invasion (LVSI) and neoadjuvant chemotherapy (NACT): IBS does not advocate APBI for patients who received NACT and with LVSI positivity. However, in the absence of other high-risk features, focal LVSI may be a relative contraindication.
Histology: All invasive and non-invasive histologies.
Multi-centricity and multi-focality: All efforts should be made to rule out multi-centricity and multi-focality (MRI or sono-mammography), as these are the absolute contraindications for APBI.
Rationale for brachytherapy boost after WBI
The conventional method of radiotherapy is to deliver 45-50 Gy of WBI, followed by a boost of 10-16 Gy. This practice has currently changed to hypo-fractionated RT in 3 weeks plus boost in cases with various risk factors. Nevertheless, large randomized trials have shown the benefit of boost irradiation [39-41]; it may be avoided in post-menopausal patients. Boost irradiation is frequently used with electrons, photons, or MIB in low-, high-, or pulsed-dose-rate treatments. Even though MIB demonstrated superior results in an EORTC trial, it is a less often practiced technique [42]. The local recurrence rate of electrons, photons, and MIB was found to be 4.8%, 4.0%, and 2.5%, respectively. Traditionally, MIB boost is performed following WBI. However, it can be done prior to WBI during peri-operative period by implanting interstitial catheters and giving the boost typically in 24-48 hours after surgery. It may have numerous advantages: 1) Catheter placement is exact and accurate, since it is done with surgical team’s direct vision and palpation of the lumpectomy chamber. This minimizes the risk of geographical errors, 2) Vascularity of the boost treatment area is preserved during the peri-operative period, in comparison with hypo-vascularity caused by fibrosis that occurs when the boost is delivered after EBRT, 3) Improved delineation of the lumpectomy cavity, 4) Avoidance of re-hospitalization and resource overburdening because the boost therapy is delivered during surgical stay, 5) Increase of patient comfort by avoiding trauma and pain.
Apart from the above-mentioned benefits, MIB boost may increase the risk of infection and delay wound healing in various patients.
Patient selection for brachytherapy boost
Even though the EORTC trial [42] demonstrated a reduced risk of ipsilateral breast tumor recurrence (IBTR) compared with WBI alone in all age groups, the absolute risk reduction was most significantly observed in younger women. IBS recommends tumor bed boost to all patients, except for post-menopausal cases who underwent BCS. The technique of boost should be tailored individually. MIB is suitable in most situations, except for a large tumor to breast ratio, and type 2 oncoplasty or higher performed.
Techniques of interstitial brachytherapy
Numerous brachytherapy techniques exist that can be apply for APBI and brachytherapy boost, each with its own advantages and disadvantages. Table 2 provides the details of the various techniques.
Table 2
Brachytherapy techniques
Surgical principles: There are two major lumpectomy techniques: open cavity and closed cavity. In the first, only the surface of the cavity is closed, allowing a seroma to form. Smitt et al. [43] proposed a cavity visualization score (CVS) based on computed tomography (CT) scans: CVS-1 (cavity not visualized), CVS-2 (cavity visualized but margins indistinct), CVS-3 (cavity visualized with some distinct margins and heterogeneous appearance on CT), CVS-4 (cavity with mild heterogeneity on CT and majority of margins distinct), and CVS-5 (homogenous appearance of the cavity on CT and all margins seen clearly). In the closed cavity technique, a full-thickness closure is performed by suturing deep and superficial layers of the surrounding breast tissue. In this instance, seroma does not form; therefore, defining the desired volume can be more challenging (CVS score, 1-3). Open cavity surgeries with seroma formation can result in larger volume CTVs and increase complications. On the other hand, closed cavity surgeries result in smaller CTVs, allowing for liberal margins [44]. In addition to open and closed cavity surgeries, oncoplastic procedures are routinely performed during BCS. On the one hand, they allow for reconstructive surgeries and better cosmesis, but on the other hand, they cause partial displacement of the original tumor tissue [45, 46]. Therefore, many radiation oncologists avoid utilizing APBI after oncoplastic surgeries. A retrospective review among 131 patients who underwent oncoplastic procedures followed by APBI was performed; only one case developed local recurrence and two patients had a different quadrant recurrence after a median follow-up of 40 months. Although the follow-up was short, it appears that APBI is safe and feasible in patients who undergo oncoplastic procedures, primarily type 1 oncoplastic surgeries [47].
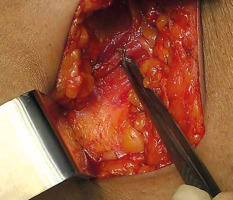
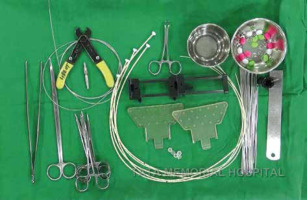
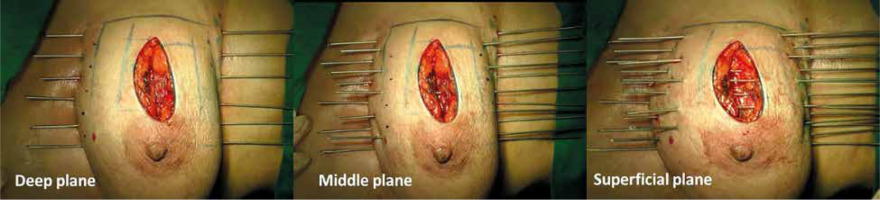
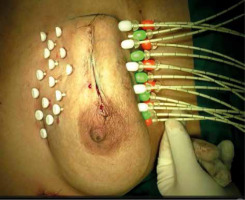
Free-hand intra-operative technique: The open free-hand technique relies on the brachytherapist ability to insert catheters or needles in an array that covers the desired volume, while maintaining a spacing that ensures a uniform dosage distribution. The extent of surgical excision is evaluated by the radiation oncologist, while the skin incision is open by probing the cavity with an index finger. Surgical clips are inserted into the cavity wall where the wall meets the base (Figure 1). Usually 4 clips are placed in the cranial, caudal, medial, and lateral wall of the cavity. Additionally, clips are placed in the center of the base within the site of the tumor. It is important to ensure hemostasis before proceeding with the procedure, and with the implant tray ready (Figure 2). Then, using a sterile marker or gentian violet (GV) paint, the area of interest as well as the entry and exit points are demarcated. If the tissue depth is larger than 1 cm but less than 2.5 cm, a double-plane is required. A third plane is added when the target tissue exceeds or equals to 2.5 cm depth (Figure 3). Smaller volumes demand tighter spacing, while bigger volumes require broader spacing. The gap between planes is 1.5-1.8 cm for multi-plane implants, while the inter-catheter separation within each plane is usually 1.5 cm. The needles are replaced with plastic tubes and fastened using buttons of different colors for easy identification of different planes (Figure 4). Basic free-hand principles include: 1) Implanted area should be larger than an area marked with ink to ensure safety margin and adequate coverage, 2) Catheter entry and exit locations should be selected at least 1 cm away from the target volume, or a source dwell will be required in the skin to avoid skin overdose that can lead to telangiectasia, 3) Ensure that the exit site of catheters is as away from the body as possible – this will reduce the transit dose. CT planning should be planned 2-3 days after implantation. Treatment can either begin after availability of histopathology report (typically 5 days after surgery), or 3 days after the procedure. After 4 fractions, histopathology is confirmed. In case of adverse pathological features, these 4 fractions are converted into a boost. In favorable histopathological report, treatment is continued further with the prescribed APBI dose.
Fig. 4
Clinical image showing the interstitial brachytherapy catheter insertion fixed with buttons of different colors
Free-hand technique after 3 to 6 weeks of surgery: While there is no significant difference in dosimetry and implant quality compared with intra-operative technique, it may not provide direct visualization of the tumor bed [48]. A pre-planning CT is performed before the actual brachytherapy procedure. Then, radio-opaque markers are placed on the skin with a margin of 2 cm to the cavity visualized either with the help of surgical clips (closed cavity) or seroma (open cavity). On the day of procedure, catheters are placed within the pre-marked target zone using a single-leader technique with or without ultrasound guidance. Superficial plane is introduced at 1 cm below the skin’s surface. When the distance between the superficial and deep planes falls between 2.5 and 3 cm, a middle plane is added [49].
CT-guided template-based technique: A CT-compatible plastic template is placed on the breast one day before implantation considering the scar position on the skin. First, the distance between template plates is measured, and their points on the skin are marked. Next, pre-implant CT imaging is performed, and the tumor cavity and clinical target volume is defined in axial slices. The patient’s imaging data are then rotated to the “needle’s eye view”, i.e., seeing in the direction of the needles in treatment planning system (TPS), and the target volume is projected onto the rendered template with the holes. Visual examination identifies the holes covering the target volume, and their coordinates are recorded. The next day, a more rigid template that is geometrically equal to the first one is placed in the same position as in the previous day, utilizing the skin marks and template parameters as guidance. Needles are then inserted into the breast using pre-determined coordinates, and replaced with plastic catheters. Another CT data set is obtained for the treatment planning, and the definition of target volume and OARs. If no acceptable target coverage is found, a few extra catheters should be implanted by hand, without the assistance of a template. Repeat CT imaging is essential for planning in this scenario [50]. A template can be used as the only guidance for APBI, since the template may lead to pressure ulcers if kept for longer time during the entire APBI. The template can however be used for a boost treatment.
Timing of brachytherapy with chemotherapy
There is paucity of data on sequencing and appropriate interval between chemotherapy and APBI. A recent study [51] investigated the effect of timing and sequencing of chemotherapy on clinical outcomes in EBC patients treated with APBI. Wound complications (WCs) were detected in 24 patients (11%), wound infections (WIs) occurred in 20 and wound dehiscence in 4 cases. In women who developed WCs, the median time between chemotherapy and APBI was 13 days, 20 days for pre-chemo APBI, and 32 days for post-chemo APBI in the absence of WCs. On multivariate analysis, less than 3-week interval between APBI and chemotherapy was the sole significant factor (p = 0.03) influencing WCs. To achieve the best cosmesis, IBS recommends a minimum of a three-week gap between chemotherapy and APBI.
CT simulation
Sharma et al. compared the dosimetric outcomes of orthogonal radiograph-based and computed tomography-based plans prepared with various optimization procedures for 18 partial breast intra-operative implants [49]. The study revealed superiority of the three-dimensional CT over conventional two-dimensional radio- graph-based planning in terms of reduced normal breast irradiation with prescribed dose and improved compliance. In CT-based dosimetry planning, inter-active graphical optimization tools enhanced conformance while reducing dose uniformity. CT is the imaging modality of choice for treatment planning, including catheter reconstruction. It is advisable to apply a slice thickness of 3 mm or less. Dwell periods inside the catheters vary; precise and unequivocal catheter numbering on the CT data set as well as labelling of the real catheters are critical during reconstruction, especially when the planning data are sent to a therapy machine. A diagram of the implant and numbering should be recorded at the time of simulation.
Catheter reconstruction
Radio-opaque markers can be put into the catheters before imaging for improved visualization. However, in most situations, the internal air in the catheters can act as a surrogate for the markers, and the reconstruction can be completed successfully with proper windowing. This may not be possible in open cavity technique, as in addition to seroma, there is generally air on top and identifying the catheter becomes difficult. Hence, we recommend that copper wires should be inserted at the time of CT simulation for precise catheter reconstruction.
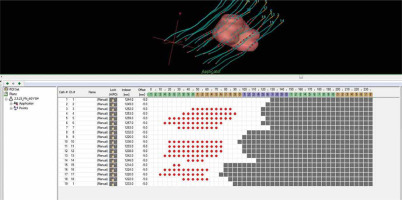
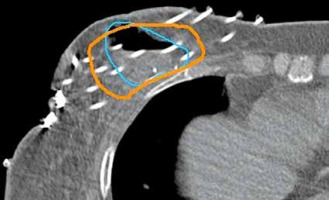
A CT-marker can reveal the first probable dwell location in the catheter, but the equivalence of intended and actual source positions must be known and validated by a measurement at least once. When no markers are utilized, the fixation button at the distal end of each catheter must be visible on CT images, since the first potential dwell position must be associated with it. Catheter reconstruction can be carried out in axial, sagittal, or coronal planes (Figure 5). Once all possible source dwell positions are reconstructed, the active source duration must be established. In the first phase, active source positions inside PTV may be defined (from surface to surface). The final configuration of active source locations is determined according to the type of optimization used as well as the resulting dose distribution and dose-volume histogram (DVH) values. If necessary, the active lengths can be extended by a few millimeters beyond the PTV, as shown in Figure 5 [50].
Target delineation
For CTV contouring, we recommend obtaining the details of pre-operative clinical examination, imaging (mammography, MRI, or ultrasound), surgical procedure (type of surgery, number, and locations of surgical clips, and position of skin scar), pathology findings, including tumor-free margins in six directions (Figure 6). The proper safety margin around the resection cavity is constantly being debated. A 15 mm uniform expansion creates the CTV from the lumpectomy cavity in the NSABP B-39; however, it is limited by the skin and chest wall [34, 52]. The total safety margins for the CTV should be defined as the sum of the size of the surgical resection margins plus the size of the added safety margins. Most of the time, the surgical resection margins around the original tumor are not symmetrical and diverge in different directions. This fact must always be taken into account while defining the target, and the pathology report must provide the tumor-free margin in all six directions. A study revealed that smaller implants had lower conformity when anisotropic margins were grown compared with isotropic margins [53]. We recommend leaving a 10 to 15 mm margin around the tumor bed as a safety net, and adapting it anisotropically based on surgical margins. In case of organs at risk (OARs), accurate delineation of the skin is important as it often predicts cosmetic outcome [54].
Optimization
Dose optimization refers to determining individual dwell duration in each dwelling position to achieve the optimum in target coverage, OARs sparing, and dose uniformity. Dose optimization techniques can assist in enhancing dose distribution, but no optimization can compensate for inadequate implant geometry. The manual editing of dwell times is the simplest optimization. Geometrical optimization (GO) is the primary method that produces a homogeneous dose distribution. The tar- get coverage by the reference dose will also be appropriate, if the catheters geometrically cover the target volume correctly. Graphic optimization (GRO) can be used to adjust the shape of isodoses locally or globally, allowing a selected isodose line to be relocated into the correct place using a computer mouse’s “drag-and-drop” capability. With GRO, the target coverage and conformance can be enhanced, but it is essential to remember that homogeneity may suffer as a result.
Dose recommendation
Various dose schedules can be considered for APBI using interstitial brachytherapy (Table 3). These include 34 Gy in 10 fractions, 32 Gy in 8 fractions, and 35 Gy in 10 fractions using bid regimen, 6 hours apart. In case of a delay in the histopathology report, this dosing strategy allows for the delivery of the first four fractions as a boost (if required). Other fractions can also be utilized. Nonetheless, we recommend that the fractionation used corresponds to a BED of 65 Gy and an EQD2 of 43.75 Gy, considering an α/β of 4. For brachytherapy boost, a total dose of 12-15 Gy delivered in 3-4 fractions, 3-3.5 Gy per fraction twice a day is recommended.
Table 3
Various studies in literature with APBI clinical results
| Study [Ref.] | Country | Dose | Median follow-up (years) | IBTR (%) | Adverse cosmesis (%) |
|---|---|---|---|---|---|
| Polgar et al. [62] | Hungary | 36.5 Gy/7 fx. | 10 | 5.9 | 19.0 |
| GEC-ESTRO [2] | International | 32 Gy/8 fx. | 5 | 1.9 | 8.0 |
| NSABP B-39 [34] | International | 34 Gy/10 fx. | 10 | 4.6 | – |
| Budrukkar et al. [38] | India | 34 Gy/10 fx. | 5 | 4.4 | 2.0 |
| Sharma et al. [79] | India | 35 Gy/10 fx. | 5 | 1.6 | 10.0 |
DVH
Implant-related dose-volume metrics, such as the volume irradiated by the prescribed dose (PD) (VPD) or 1.5 times the PD (V1.5 × PD) can be computed without taking any defined volumes into account. The dose non-uniformity ratio (DNR) specified as V1.5 × PD to VPD, defines the dose distribution homogeneity. The lower the DNR, the more uniform the dosage distribution. Dose homogeneity index (DHI) can be developed to supplement DNR. By definition, DHI = (VPD V1.5 × PD)/VPD, where DHI is 1 – DNR. Table 4 summarize the most frequently used DVH parameters in interstitial breast brachytherapy. Additionally, on the post-implant CT scan, the dose distribution must be analyzed in 3 different angles (i.e., axial, coronal, and sagittal) to confirm the primary dose constraints and avoid the confluence of two successive V200 isodoses, with a V200 isodose diameter greater than 10 mm [55-58].
Table 4
Dose-volume constraints for target and organs at risk (OARs)
Post-implantation care and catheter removal
A course of routine antibiotics is prescribed from the day of implant insertion. During the course of treatment, caution should be taken that the buttons are not pushing too hard on the skin and are not too loose, in order to avoid ulceration and formation of chronic skin marks, such as acromia or skin necrosis in the future. In the event of visible or suspected changes in the breast volume or in the position of plastic catheters, a CT scan needs to be performed, and if necessary, re-planning must be considered. The temporal variation in the CTV during the course of APBI after open cavity surgery was shown to have a significant impact on the coverage and conformity [59]. On removal, mild to moderate compression is applied to control seroma and blood oozing from the entry or exit points. The points may be sealed with tincture benzoin. The patient is sent home with an antimicrobial dressing that is removed after 2-3 days.
Literature on MIB-based APBI and boost
Brachytherapy techniques, which can be used intra-operatively or post-operatively have the most reliable APBI data. Unfortunately, interstitial brachytherapy is available only in well-established facilities, because it is highly dependent on the experience and skills of the treating physicians. Nevertheless, interstitial brachytherapy has so far produced impressive results.
Strnad et al. and Polgár et al. [60, 61] conducted a study, in which 258 patients with EBC after BCS were randomly assigned to either WBI or APBI delivered twice per day. The actuarial rate of local relapse was non-inferior after 5 years, with 3.4% for WBI and 4.7% for APBI, and after 10 years, 5.1% for WBI and 5.9% for APBI (p = 0.50 and p = 0.77, respectively). In terms of esthetics, the findings supported APBI, with an 81% rate of excellent to good cosmetic results for APBI against 63% for WBI (p = 0.01). The 20-year updated results of this trial [1] further validated that APBI yields long-term local control and survival comparable with those achieved with standard WBI.
In another trial, WBI was shown to be 7% more likely than APBI to cause grade 3 radiation dermatitis (p = 0.0001). WBI caused acute toxicity in 86% of patients, but APBI in 21% (p = 0.0001). APBI had somewhat higher rates of moderate hematoma and mild breast infection than WBI (20% vs. 2%). Additionally, WBI had a 5-year local recurrence rate of 0.92% (range, 0.12-1.73%), while APBI presented 1.44% of the same (range, 0.52-2.38%) (p = 0.42) [62-64].
The NSABP B-39/RTOG 0413 trial randomly assigned 4,216 EBC patients receiving WBI (50 Gy in 25 fractions with a sequential boost) or APBI (34-38.5 Gy in 10 fractions given twice daily with brachytherapy or EBRT), following BCS. There were no statistically significant differences in DFS and OS rates between APBI and WBI [34]. While grade 2 toxicity was marginally lower in the APBI group (44% vs. 59% for WBI; not significant), grade 1 and 3 toxicity were slightly greater in the APBI group (40% vs. 31% for WBI, and 9.6% vs. 7.1% for WBI, respectively; not significant). Nonetheless, over 10 years, the absolute variation in IBTR rates was encouragingly small, at 1% (4.8% for APBI vs. 4.1% for WBI) [26, 34].
The afore-mentioned randomized trials’ results resemble prior single-arm studies utilizing interstitial brachytherapy. Even though the NSABP B-39 trial allowed both EBRT and brachytherapy, most patients were treated with EBRT. Local recurrence rates are slightly higher, ranging from 5.2% to 15% after over 10 years of follow-up, showing outstanding cosmetic outcomes [65-69].
On the contrary, in a recent meta-analysis, the local recurrence rate for APBI was significantly higher than that for WBI, though without any difference in DFS or OS rates. In the sub-group analysis, APBI was equivalent to WBI if performed in a TPS. This meta-analysis included studies with intra-operative RT (IORT) performed without a TPS, and may have resulted in inferior outcomes [70].
Budrukkar et al. investigated the clinical results of EBC patients treated with MIB-APBI utilizing 3-dimensional CT (3D-CT) planning. A dose of 34 Gy in 10 fractions was delivered in 5 days using high-dose-rate (HDR) brachytherapy. After 5 and 7 years, LC were 97% and 92%, respectively. Her-2-positive was the sole predictive factor associated with an increased risk of LC (p = 0.01). Five- and seven-year DFS and OS rates were 93% and 84%, and 97.5% and 89%, respectively. Good to excellent cosmetic outcomes at the last follow-up were observed in 77% of patients. It was concluded that MIB performed using 3D-CT produced excellent long-term outcomes and good to exceptional cosmesis [38].
Recently, Wadasadawala et al. conducted a matched-pair analysis to compare early cosmetic outcomes after WBI and APBI [71]. Sixty-four APBI patients were matched with 99 WBI patients from a 320 cases cohort. At a median follow-up of 25 months, cosmetic outcomes were significantly better with APBI compared with WBI. Individual metrics that were considerably superior in the APBI group included breast size and shape [72]. Another study showed that each percent of an increase in Dmax increased the probability of development of grade 2 skin marks by 5% [73]. Hence, it is recommended to restrict the dose to the catheter insertion sites as low as possible.
Fat necrosis (FN) is a known complication of breast brachytherapy, and its incidence ranges from 2% to 52% [9, 74-76]. In a retrospective research [77], clinical, radiological, and pathological rates of FN were investigated in 171 women treated with APBI. At a median follow-up of 48 months, a crude FN rate of 11.6% was observed.
Numerous guidelines exist for APBI, but none can differentiate LC (main APBI end-point) among its risk groups [24]. In a study from India [24], 240 women underwent MIB APBI. A prospectively kept database was utilized to compare long-term clinical outcomes stratified by risk groups indicated by GEC-ESTRO, ABS, and ASTRO recommendations. At a median follow-up of 114 months, the overall group’s 10-year LC, DFS, and OS were 90%, 81%, and 83.5%, respectively. There was no statistically significant difference in LC rates between risk categories according to ESTRO, ASTRO, modified ASTRO, and ABS standards. IBS recommendations for APBI is an attempt to stratify risk groups better, so that a clinically meaningful classification is established.
Sharma et al. studied the efficacy of peri-operative HDR interstitial brachytherapy boost in 100 patients with EBC. During BCS procedure, a brachytherapy implant was placed. The boost therapy was initiated on the third post-operative day, with a prescription dose of 15 Gy in 6 fractions over 3 days. Three weeks later, WBI was initiated at a prescribed dose of 50 Gy. At a median follow-up of 52 months, no patient had a local recurrence. The 5-year OS and DFS rates were 86% and 77%, respectively. Eleven individuals developed acute toxicity, four had wound problems, and seven suffered from grade 3 skin toxicity. Nine of the eleven patients had breast volumes exceeding 1500 cc. Except for the breast volume (> 1500 cc), no patient’s dosimetry-related factor was found associated with acute toxicity in a statistically meaningful manner [78, 79].
Conclusions
Brachytherapy continues to be an established treatment in the management of EBC. Various trials have proved that brachytherapy-based APBI is a successful treatment in appropriately selected EBC patients. Moreover, MIB provides the best dosimetry for boost treatment (whenever indicated) compared with other techniques. As the breast brachytherapy is an underutilized therapy in the Indian context, this article will hopefully encourage the readers to use its clinical potential in appropriately selected early breast cancer patients.