Introduction
Biliary atresia (BA) is one of the most serious hepatobiliary diseases. Biliary atresia leads to severe conditions with obstruction of both intrahepatic and extrahepatic bile ducts from the fetal period to before and after birth. The incidence of BA has been reported as 1/10,000 live births in Japan. Obstruction of the biliary tract leads to intrahepatic biliary congestion, and continuation of obstruction causes biliary liver fibrosis. Progression of intrahepatic disease results in complications of portal hypertension and advanced biliary cirrhosis. The natural course of BA is that patients grow relatively well until approximately 2-3 months of age, but after 4 months, they develop nutritional disorders, anaemia caused by liver cirrhosis, hypoproteinaemia, ascites, and various fat-soluble vitamin deficiencies. Progressive hepatic failure then leads to death at 1-2 years of age.
The first Kasai portoenterostomy (KP) for BA was performed in 1959, and widespread liver transplantation began in the 1990s in Japan. Advances in surgery and postoperative management have greatly improved the outcome of BA. In 2020, the Japanese Biliary Atresia Registry (JBAR) reported that the overall survival rates of patients with BA were 86.7% and 83.2% at 20 and 30 years, respectively [1]. In the JBAR study, the jaundice clearance rate for all enrolled patients was 61.7%, and the native liver survival rate was 43.0% at 20 years postoperatively and declined to 36.6% at 30 years postoperatively. However, when KP was performed before 30 days of age, the jaundice clearance rate was 72.7%, and the native liver survival rate was 56.2% at both 20 and 30 years after surgery.
Furthermore, according to the JBAR, native liver survival rates at 20 and 30 years postoperatively decreased as the age at initial surgery increased (56.2% and 56.2% within 30 days; 47.8% and 38.2% at 31-60 days; 41.1% and 36.3% at 61-90 days; and 33.6% and 28.1% at 91-120 days, respectively). Therefore, KP during the neonatal period considerably affects both the jaundice clearance rate and the long-term native liver survival rate. However, even in 2020, the average age when patients had KP performed was 52.1 days, and the current situation is that 16.0% of patients receive an operation within 30 days. If this average age can be lowered, it may improve the native liver survival rate and the quality of life of affected children. This improvement of the native liver survival rate may also reduce medical costs by reducing the rate of liver transplantation.
In Japan, the Maternal/Baby Health Handbook is issued to every pregnant woman by the Japanese Ministry of Health, Labour, and Welfare to record details of the pregnancy, delivery, the baby’s health, assessment of growth/developmental milestones, and vaccination history. The stool colour card (SCC) developed by Matsui et al. [2] (Fig. 1) has been included in the handbook to screen for BA nationally since 2012. To diagnose this disease in the neonatal period, children must be referred to a specialist, such as a paediatrician or a paediatric surgeon, for a thorough examination. Therefore, parents or guardians should report an abnormal stool colour, or midwives or other healthcare professionals should take note of abnormalities in the colour of stools during obstetric examinations. However, this method of detecting SCC is highly subjective. Moreover, there is a high possibility of missing patients with more subtle manifestations of BA. In the JBAR study, among 31 patients younger than 30 days with BA and with a record of SCC, 41.9% had a stool colour grade of 4 [1], even though the SCC recommends only seeing a specialist if the stool is graded as 1-3. This finding suggests that many patients have been missed by SCC screening alone. We consider that a more objective test is required to identify patients with BA who are among those whose stool colour is determined to be grade 4 in the neonatal period.
Fig. 1
Stool colour card used for nationwide universal screening for biliary atresia in Japan. Colour 1: white, colour 2: cream, colour 3: shortbread, colour 4: mustard, colour 5: tan, colour 6: caramel, and colour 7: khaki.
Instructions: Check your baby’s stool colour in a well-lit area and compare it with the colours on the card. If the stool colour is similar to nos. 1-3, or if the stool colour was similar to nos. 4-7, but is now closer to nos. 1-3, seek medical advice. Record the stool colour at least three times: at 2 weeks, 1 month, and 1-4 months of age

Indocyanine green (ICG) is an agent that is rapidly and wholly bound to albumin in the blood, and is selectively taken up by the liver and excreted in bile [3]. Extrahepatic excretion of ICG by the kidneys and intestinal circulation has also been ruled out [3]. Therefore, most ICG is thought to be excreted only in bile. The ICG test has been clinically applied as a liver function test to examine hepatic excretion capacity and other parameters. ICG is detected with a fluorescent observation camera. In recent years, ICG has also been used in various diagnostic and therapeutic applications, such as detection of intracardiac left-to-right shunting, detection of sentinel lymph nodes in breast cancer, and surgery for brain aneurysms [4-6]. Therefore, we hypothesised that ICG is not excreted in bile duct obstructive diseases such as BA, and that it should not be observable by fluorescence in stools.
In the present study, in order to develop an objective, simple, and less invasive screening method to detect patients with BA during neonatal checkups, we used the bile duct ligature (BDL) rat model as an extrahepatic biliary obstruction model and monitored the secretion of intravenously administered ICG in the stools. The results revealed that the method is indeed promising, while also indicating that some amount of ICG can be secreted by an additional route that does not involve the bile duct.
Material and methods
Animal model
Animal experiments were performed using 8-week-old Slc:SD male rats (283 ±23 g, median 280 g) purchased from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan). The rats were kept individually in cages and housed under the following conditions: light-darkness cycle 12 hours light/12 hours dark, temperature 20-26°C, humidity 40-60%, ventilation changes 6-15 times/h, and airflow velocity 13-18 cm/s. They were allowed free access to water and normal rodent chow (MF; Oriental Yeast Co., Ltd., Kyoto, Japan). All stools were spontaneously excreted and we did not use faecal induction measures such as enemas. As the amount of food intake, frequency of defaecation, and the amount of faeces vary from case to case in the clinical setting in human neonates, the food intake and the amount of faecal output were not regulated in the rats of this study.
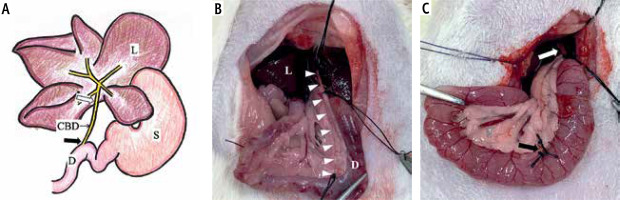
They underwent laparotomy under inhalation anaesthesia with 1.5-2.5% isoflurane. The common bile duct was ligated and dissected at two points near the hepatic portal and papillary region to create a biliary obstruction model (Fig. 2). This biliary obstructed model was designated as the extrahepatic biliary obstruction group (group O, n = 9). The unaltered group was designated as the control group (group C, n = 6).
Fig. 2
Biliary obstruction model scheme. A) The upper abdominal schema of a rat, and B, C) images of the actual experiment. A) This illustration indicates the typical hepatic portal part of a rat. L – liver, D – duodenum, S – stomach, CBD – common bile duct. A white arrow (➯) indicates near the hepatic portal, and a black arrow (➨) indicates near the papillary region. B) The common bile duct is indicated by the white darts (ᐅ) from the hepatic portal to the papillary region. C) Dissection was completed at two points near the hepatic portal (white arrow; ➯) and the papillary region (black arrow; ➨)
Administration and observation
Indocyanine green (0.5 mg/kg; Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan) was administered through the caudal vein between 9 and 11 am; group O received ICG at the end of surgery. Stools excreted during particular time frames were collected at the indicated times after administration: for example, the stools collected at 3 hours after administration were stools excreted between 0 and 3 hours after administration. Basically, all stools in the cage were collected, but if there were too many stools to fit into the collection tube, the stools in the cage were collected randomly until the collection tube was full. The collected stools were stored in a cool (4°C), dark place.
Indocyanine green in the collected faeces was observed by fluorescence using the Photodynamic Eye (PDE) system (Hamamatsu Photonics, Hamamatsu, Shizuoka, Japan) in a black box. The excitation and the emission wavelengths of the PDE system were 760 nm and 845 nm, respectively. Stools excreted by the same animal within a specified time frame showed an approximately even fluorescence.
Fluorescence was recorded by video and clipped as an image file (JPG) using the Windows 10 snipping tool. The presence or absence of fluorescence was compared as a qualitative test and lightness as a quantitative test. Lightness was measured using the following website: https://www.color-site.com. Lightness was expressed as 0-100%, with 0% lightness as black, 100% as white, and the intermediate (50%) as a pure colour. The quantification point was the point that showed the highest fluorescence in the observed stools. If no fluorescence was observed, a central point in the tube where the stools were accumulated was used as the quantification point.
Statistical analysis
Statistical analysis was performed with Stata 17.0 (StataCorp, Texas, TX, USA). The Wilcoxon rank sum test was used to analyse the data between the two groups, and the differences were considered significant at p < 0.05. Receiver operating characteristic curves were also used to detect cut-off values.
Results
Qualitative test: fluorescence
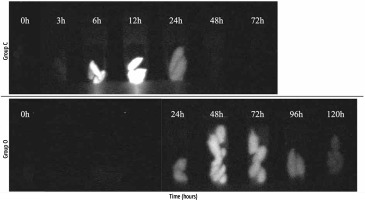
The qualitative test results are shown in Figure 3. In group C, stools were collected at 3, 6, 12, 24, 48, and 72 hours. In group O, stools were collected 24, 48, 72, 96, and 120 hours after surgery because the rats could not stably excrete within 24 hours after surgery and the collection of their excrements was too complex. Except for the stools within 24 hours in group O, we could collect stools for each of the selected time frames from all of the individual rats in both groups. In group C, stools became fluorescent 6 hours after ICG administration, and fluorescence disappeared at 48 hours. Group O stools became fluorescent at 24 hours, and the fluorescence continued at 120 hours without loss of fluorescence.
Quantitative test: lightness
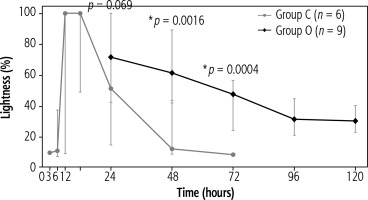
The results of the quantitative test are shown in Figure 4. The peak lightness in group C was reached during 6-12 hours (6 hours: the median, 100% [inter-quartile range (IQR), 100-100]; 12 hours: median, 100% [IQR, 100-100]), and from 48 hours onwards, the values were almost the same as those before administration (48 hours: median, 11.75% [IQR, 10.4-13.4]; 72 hours: median, 7.8% [IQR, 7.8-7.8]). However, group O recorded the highest value (median, 71.4% [IQR, 57.6-89.4]) at 24 hours. After 24 hours, lightness in group O showed a slower decline than that in group C, and even after 120 hours, it did not decrease to the values observed after 48 hours in group C (120 hours: median, 29.8% [IQR, 26.7-33.7]). When we compared the time points of 24, 48, and 72 hours, the lightness at 48 and 72 hours showed significant differences between the groups (p = 0.0016 and p = 0.0004, respectively). The cut-off values at 48 and 72 hours, which were significantly different, were 41.6% (sensitivity: 100%, specificity: 100%) and 23.5% (sensitivity: 100%, specificity: 100%), respectively.
Fig. 4
Results of the quantitative lightness test. The points indicate median values, and the bars indicate minimum to maximum values. In group C, peak lightness occurred at 6-12 hours, and the values were almost the same as those before administration from 48 hours onwards. In group O, the highest value was at 24 hours, and values did not decrease to the values observed after 48 hours in group C, even after 120 hours. There were significant differences at 48 and 72 hours between the two groups (p = 0.0016 and p = 0.0004, respectively)
Discussion
Our study showed two important findings. The first finding involved the ICG excretion pathway and the other involved the potential use of ICG fluorescence for cholestasis disease screening. ICG was thought to be selectively taken up by the liver from blood and excreted only in bile, with no excretion from the intestinal circulation or kidneys [3]. However, despite the fact that the rats’ bile ducts had been thoroughly dissected, which should have prevented bile from flowing into the intestinal tracts of these animals, we unexpectedly observed ICG fluorescence in the faeces of group O. Although ICG has been used as a test to measure liver excretion capacity, our results indicate that bile is not the only route for its excretion. Previous studies have reported that ICG is not excreted in the urine, which suggests that ICG flows out into the intestinal tract owing to shedding of the intestinal mucosa, microbleeds, or excretion from body fluids such as lymph fluid. We have observed mild elevation of bilirubin and light-yellowish colouration of the stools in clinical situations, even though BA is an extrahepatic obstructive disease of the bile duct. These clinical phenomena also support the view that bile flows from the narrowed bile duct in small amounts and through other routes such as lymph fluid. However, we consider that it is difficult to determine lymphatic excretion of ICG due to the mixing of blood and lymph fluid when tissue samples such as from the intestine are isolated, and we did not test this hypothesis in the present study. Although it is difficult to prove the hypothesis by using tissue samples, it may be possible to prove it by dynamic observation using two-photon laser scanning microscopy (TPLSM). The TPLSM microscope allows non-invasive fluorescence observation of the inside of biological tissues and is a suitable optical instrument for bioimaging. In previous studies involving one of the co-authors, live mice underwent laparotomy under anaesthesia and the intestinal tract was withdrawn from the body, and blood flow in the intestinal wall was observed from the intestinal serosal surface using TPLSM [7]. Using TPLSM in this way, we believe that it may be possible to observe in real time the moment of leakage of fluorescently observable ICG from the intestinal blood vessels or lymph vessels into the intestinal lumen. Therefore, we need additional experiments to determine the excretion pathway of ICG in group O, and it might allow us to develop a new treatment that could delay or avoid surgery.
The pathogenesis of BA is still unknown. However, in our study, ICG fluorescence in stools was continuously observed for more than 48 hours after ICG administration in group O, while it disappeared at 48 hours in group C. Our results suggest that infants with delayed ICG excretion and fluorescence require detailed testing for suspected BA or other cholestasis diseases. Furthermore, our lightness test provides a numerical value, which would be more objective than the SCC.
In this study, ICG was administered from the caudal vein. However, in a clinical situation, a less invasive approach than intravenous injection of infants would be preferable. Intradermal administration of ICG is used in lymphangiography [8]. If intradermal administration proves to be sufficient, this technique would be more accessible and could be administered by nurses at the obstetric level. In addition, research has recently been conducted on oral ICG administration. Therefore, the administration of ICG is expected to become a more straightforward and less invasive testing method in the future [9].
Cross-reactions with iodine have been noted with ICG, and allergic reactions have been reported, but are extremely rare [10]. Other serious side effects are infrequent with this drug. However, a reduction in the dosage of ICG to the minimum necessary for detection should be investigated to improve the safety profile of this method. In this study, we used a concentration (0.5 mg/kg) that is widely used in clinical practice, but evidence suggests that a concentration of 1/5 (0.1 mg/kg) would be sufficient as a test to evaluate liver function [11]. Therefore, the administration methods and appropriate concentration of ICG should be further investigated in the future.
Our study has a limitation. We conducted our experiments using an original BDL model as the extrahepatic bile duct obstruction model. The BDL animal model was developed by Cameron and Oakley in 1932 and consists of ligation or excision of the common bile duct [12]. However, even though BDL is a useful model for studying complications of BA, it is not a model of complete BA and may not perfectly show the pathogenesis of BA, since both the intrahepatic and extrahepatic bile ducts in patients with BA are closed. Therefore, the rat model of extrahepatic biliary obstruction used in our study may have shown different bile excretion pathways to those in the BA rat model. Before proceeding to clinical trials, the next step is to conduct additional experiments in the BA rat model for further investigation.
Our study is the first to suggest that monitoring ICG excretion in stools may contribute to BA diagnostics. However, it is only a preliminary experiment in rats and there is no convincing evidence yet that this method can be useful in human infants. In the future, we hope to expand our animal studies and, depending on the results, possibly progress to clinical trials in human infants.
In summary, in a rat BDL model, ICG is excreted into the gastrointestinal tract through a route other than the bile duct. However, the excretion rate is slower than in the control, indicating the potential of ICG for screening for biliary congestive disease in the neonatal period. Subsequent detailed testing could then be used to confirm a diagnosis of biliary congestive disease.