Introduction
Natural killer cell function
Natural killer (NK) cells are a subset of mononuclear cells that play an important role in both innate and adaptive immune responses. NK cells are morphologically described as large granular lymphocytes (LGL) identified by the lack of CD3 (T-cell receptor) and surface expression of CD16 (Fcγ III receptor) and CD56 antigens [1]. By an assessment of CD56 and CD16 on the surface, NK cells are divided into two major subsets. The NK1 subset presents low expression of CD56 and high expression of CD16 (CD56dimCD16+). NK1 cells comprise 90% of all NK cells in circulating blood and have stronger ability to kill target cells. Cells of the second subset, NK2, have higher expression of CD56 but they lack the CD16 receptor (CD56brightCD16–). This population produces a greater amount of cytokines, such as interferon (IFN)-γ, compared with NK1 [2, 3].
The major biological function of NK cells is to recognize and kill tumor-transformed or virus-/bacteria-infected cells through the spontaneous cytotoxic effect. This effect is mainly mediated by cytotoxic granules containing sets of lytic enzymes (perforin, granzyme, TIA) and is regulated through the balance of stimulating and inhibiting signals, which are received by the NK cells through their receptors. Ligands for those receptors are mostly MHC class I molecules. When expression of MHC class I molecules is decreased, NK cells recognize the cell as a target and trigger cytotoxic mechanisms [4].
Cytotoxic abilities of NK cells can be used as one of the indicators of immune system efficiency. Decreased NK cytotoxicity may lead to immune dysfunctions and some diseases. Amongst the most serious systemic syndromes related to impaired NK functions hemophagocytic lymphohistiocytosis (HLH) is one of the most life-threatening conditions. HLH may be divided into two types: familial, which develops as a result of genetic mutation related strictly to NK function (FHL) or linked with other genetic diseases, e.g. Griscelli syndrome type 2 or Chediak- Higashi syndrome [12]; and acquired, resulting from viral infections, rheumatic diseases or other factors. If HLH is suspected the patient must be diagnosed in a special diagnostic center and his treatment needs to be monitored in specialist laboratories [13]. Sometimes samples are delivered from distant places, which is time consuming and may affect the results of activity tests.
The cytotoxicity test is a major and commonly used tool for testing NK activity. The long-time gold standard for assessing cell-mediated cytotoxic activity of NK cells is the chromium release assay (CRA). CRA involves radioactive labeling of target cells with 51Cr and co-incubation with effector cells (K572, line of erythroleukemia cells). Although a number of non-radioactive assays have been created, most of them are based on the direct interaction between effector and target cells. For that reason, good quality of cells used in the test is crucial for credibility of the final results. The cytotoxic activity test can be carried out on isolated peripheral blood mononuclear cells (PBMC) or separated NK cells. Taking into consideration a good condition of the effector cells and previous suggestions that blood cells may act differently depending on the anticoagulant used for blood collection [7], the type of anticoagulant used for prevention of blood clotting should be carefully chosen. Anticoagulants commonly used for blood testing are sodium citrate, K2EDTA and heparin.
Anticoagulants
K2EDTA (ethylenediamine tetraacetic acid)
K2EDTA is one of the most commonly used anticoagulants. It prevents blood from clotting through the binding of calcium ions. Calcium ion binding is convertible since metal complexes are created. Thus bound ions are able to exhibit diminished reactivity. Also other metals (II/second oxidation degree), e.g. copper, magnesium or iron, can be chelated in a 1 : 1 ratio by amino acid groups from K2EDTA. By this characteristic, K2EDTA enables enzymes which have those metals in their active center. One calcium-dependent enzyme is phospholipase C. This enzyme contributes to many cells’ activities, e.g. intracellular signaling, by producing 1,2-diacyloglicerol and inositol 1,4,5-trisphosphate, ion transportation and vesicle formation [8]. K2EDTA also prevents oxidation of cholesterol and unsaturated fatty acids. Moreover, K2EDTA provides protection of labile molecules such as cytokines and hormones [9].
Sodium citrate
The second well-known anticoagulant is sodium citrate. This anticoagulant has similar properties to K2EDTA. Nevertheless, citrate chelates calcium ions by forming constant complexes. Not only calcium ions are bound by citrate, but also magnesium and zinc ions. Moreover, there are significant differences in total white blood count and total lymphocyte count between sodium citrate and EDTA. It has been reported that those parameters are decreased in blood samples drawn onto sodium citrate [10].
Heparin
Another anticoagulant used commonly in laboratory diagnostics is heparin. This mucopolysaccharide acts through binding with antithrombin and increasing its activity. Heparin is also able to bind with albumin, thrombin, von Willebrand factor and fibrinogen. Moreover, heparin stimulates release of some enzymes from platelets and granulocytes, e.g. elastase, lactoferrin, and myeloperoxidase. Heparin is able to inhibit the function of P- and L-selectin and has an affinity for platelet factor 4 and some other chemokines. Heparin can also inhibit the release of membrane-bound calcium mediated by the IP3 pathway due to blocking the binding of IP3 to its receptor. This results in higher intracellular calcium concentrations [11].
Aim of the study
The purpose of this study was to compare results of the cytotoxicity assay between samples taken from the same patient but with three different anticoagulants to standardize the diagnostic method. For this purpose we assessed and compared the effect of heparin, K2EDTA and sodium citrate on the results of the cytotoxicity test, to evaluate which anticoagulant is the most appropriate for the tests. Moreover, we wanted to assess the influence of the time of blood storage on the results of the cytotoxicity assay.
Material and methods
Whole venous blood for this study was purchased from the Public Blood Bank in Warsaw.
Peripheral venous blood from twelve healthy donors 19 to 25 years old, both males and females, was drawn into three tubes with different anticoagulants: heparin, K2EDTA and citrate.
The flow cytometry cytotoxicity test was performed twice on the same blood sample at two time points. The first procedure was started one hour after blood drawing, and the measurement was done within 6 hours. The second procedure was started after 24 hours storage of the whole blood samples at room temperature, and the measurement was done within 6 hours.
Flow cytometry cytotoxicity test
Peripheral blood mononuclear cells were separated by density gradient centrifugation on Ficoll (Sigma-Aldrich), washed three times in 0.9% NaCl solution and suspended in RPMI medium supplemented with 10% FBS in a final concentration 4-6 million cells/ml.
The human erythroleukemia cell line K562 was used as an NK cell activator. Cells were cultured in RPMI medium (RPMI-1640 Medium, SIGMA-ALDRICH) with supplementation of 10% fetal bovine serum at 37°C under 5% CO2. For the test K562 cells were stained with DiO (3,3’-dioctadecyloxacarbocyanine perchlorate) fluorescent dye for 30 minutes, washed twice with 0.9% NaCl solution and once with RPMI medium, then suspended in RPMI medium in a final concentration of 1.2 million cells/ml. The test was performed in a 96-well plate for each anticoagulant (Table 1).
Table 1
Scheme of reactions for blood samples with three different anticoagulants
| Heparin | Sodium citrate | K2EDTA |
|---|---|---|
| Control K562 | Control K562 | Control K562 |
| Control PBMC | Control PBMC | Control PBMC |
| Test | Test | Test |
The 96-well plate was centrifuged (1200 rpm for 5 minutes) and incubated for 4 hours at 37°C under 5% CO2. After incubation cells were stained with propidium iodine (5 µl) for 30 minutes at 37°C under 5% CO2 and analyzed in a flow cytometer.
Flow cytometry analysis
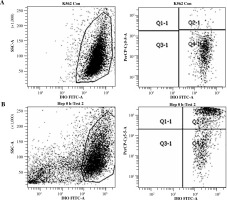
A two-parameter cytogram, log (FL1) vs. log (FL3), was plotted to discriminate the different cell types in the experiment. Acquisition was run to the final number of 10,000 events presenting DiO fluorescence.
Live target (K562) cells showed only DiO18 fluorescence, while dead target cells showed both DiO18 and PI fluorescence. Special gating was applied to obtain the number of DIO stained cells which also presented PI fluorescence. This population was considered to be killed by NK cells in a cytotoxic reaction (Fig. 1). The specific lysis index was presented as the percentage of dead target cells (cultured with effector cells) with subtracted dead target cells (cultured without effector cells).
Results
Results of cytotoxicity tests were presented as a percentage of target cells killed by effector cells throughout the 4 hours of incubation. Spontaneous mortality of target cells, assessed in the control tube, was subtracted from the test results. The first cytotoxicity test was carried out within 6 hours from blood donation. The subsequent test was performed after 24-hour storage of the whole blood sample at ambient temperature. The mean values of cytotoxicity tests performed on the day of blood donation were comparable for all anticoagulants (Fig. 2). No statistically significant difference was noted.
Fig. 2
Values of cytotoxicity measurement in blood taken from 12 patients, with three different anticoagulants. Test performed within 6 hours after blood donation. Values of cytotoxicity in the table and the chart are presented as a percentage (%)

Despite the fact that all the samples were taken from healthy volunteers, the standard deviations of cytotoxicity results were quite high. There was no significant difference in the value of cytotoxicity between all three samples. However, the highest mean values were obtained in samples with sodium citrate.
In all the cases the cytotoxicity decreased after 24-hour storage of the whole blood sample at ambient temperature (Fig. 3). The mean drop in cytotoxicity results was substantial for all anticoagulants: 76% for heparin, 67% for sodium citrate and 70% for EDTA.
Fig. 3
Values of cytotoxicity measurement in blood taken from 12 patients, with three different anticoagulants. Test performed after 24 hours from blood donation. Values of cytotoxicity in the table and the chart are presented as a percentage (%)

There was a statistically significant decrease of the value of cytotoxicity in all samples after 24 h storage (p < 0.01) (Fig. 4).
The analysis of the scores of cytotoxicity in samples with different anticoagulants demonstrated that cells stored with citrate 3.2% presented the lowest cytotoxicity drop in comparison to heparin and K2EDTA.
Conclusions
Time of the sample storage has a great impact on NK cells’ cytotoxicity, leading to its significant decrease.
Sodium citrate is the most appropriate anticoagulant for tests performed 1 hour after blood taking and after 24 hours of blood storage.
Discussion
It has been reported that anticoagulants commonly used in laboratory medicine may have a significant impact on the results of some assays [14]. Although the cytotoxicity test is a major and commonly used tool for evaluation of NK cell function, there is no consensus across laboratories as to which anticoagulants should be used for blood protection. Standardization in blood preparation and storage is required to enable comparison of results obtained in different laboratories. Another factor which should be taken into consideration regarding cytotoxicity test results is the time of sample delivery to the laboratory. Since the method of NK cytotoxicity measurement is quite complicated and requires specific laboratory equipment, it is available only in specialized immunological laboratories.
In this study we compared cytotoxicity results measured in blood samples drawn with three most popular anticoagulants at the time of blood donation and after 24 hours of storage at ambient temperature. According to our findings, sodium citrate seems to have the smallest impact on the results of the cytotoxicity test.
There may be a few reasons for the differences in the cytotoxicity results regarding anticoagulants added to the blood sample. The capacity of NK cells for spontaneous cytotoxicity against target cells may be modified by the anticoagulant used. There is some scientific evidence indicating that different anticoagulants may affect the process of PBMC isolation [7]. In one of the studies blood samples with heparin and citrate were analyzed with an ultrasonic scanner. Heparinized blood revealed considerable cell and platelet aggregation. This phenomenon was less visible in blood sample with citrate [20]. We did not notice such an influence in our study, but an impact of these substances on the number of NK cells after isolation in the total population of PBMC cannot be excluded. A correlation between the percentage of NK cells in circulation and results of the cytotoxicity test was previously found [19]. The separation technique used in our study (with Ficoll Sigma-Aldrich) can remove not only granulocytes to acquire PBMC but also dead lymphoid cells from the specimen (including NK cells), which may have contributed to decreased cytotoxicity after 24 hours [15].
It is a common knowledge that Ca2+ is a key metabolic factor for NK function including spontaneous cytotoxic reaction against target cells. It was recently reported that cytotoxicity of NK cells is strongly dependent on Ca2+ influx from the extracellular medium [21]. Later finings led to identification of Orai1 channels which are required for exocytosis and target cell killing by primary human NK [22]. Taking into consideration that reactivity of cells and intracellular signaling depends largely on the concentration of calcium ions, the use of EDTA and citrate, which bind free calcium from the environment, may affect cell activity. Studies on neutrophils showed that the intracellular concentration of calcium ions was considerably lower in samples with EDTA than in heparin or citrate. Activation of neutrophils measured by the assessment of oxidative burst was also lower in samples with EDTA than those with citrate and heparin [7]. It is in line with our observations, where cytotoxicity was the highest in samples with citrate and the lowest in those with EDTA.
Anticoagulants may also affect NK reactivity through modification of interleukin secretion. It was reported that interleukin (IL)-1 concentrations in PBMC separated from blood with EDTA were lower than those with citrate or heparin [16]. Although we observed higher cytotoxicity in samples with citrate and heparin than with EDTA, it has been found in previous studies that granzyme b levels were higher in blood samples anticoagulated with EDTA than with heparin [17]. Although we did not measure IL-1 concentration in our study, its impact as a proinflammatory cytokine on NK cell activity cannot be excluded.
The influence of blood sample storage and type of anticoagulant (heparin and EDTA) on NK cell activity in whole blood was also analyzed by Son and collaborators. They observed that NK cytotoxicity was higher in heparinized blood, which is similar to our results. They also evaluated NK activity in freshly drawn blood and blood stored for 18 hours. In both cases heparin was a better anticoagulant for this test [18].
According to our findings, and in line with other studies on this subject, sodium citrate seems to be the best anticoagulant for assessment of NK cytotoxicity. However it should be underlined that long blood sample storage before testing has a significant influence on the results, observed as decreased cytotoxicity.