Purpose
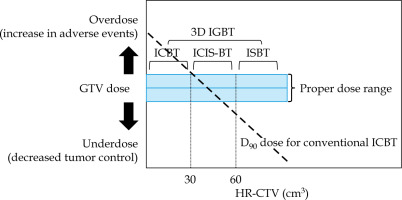
Chemoradiotherapy, including external beam radiotherapy (EBRT) combined with brachytherapy [1-3], is a standard treatment for patients with locally advanced cervical cancer. Intra-cavitary brachytherapy (ICBT) with tandem plus ovoids or rings is the most commonly treatment used. However, conventional ICBT delivers treatment dose to specific reference points regardless of the target size or shape. American and European brachytherapy groups have established guidelines and recommendations for magnetic resonance imaging (MRI)-based ICBT [2-6]. To overcome this limitation, image-guided adaptive brachytherapy using three-dimensional image information with combined intra-cavitary and interstitial brachytherapy technique (ICIS-BT) was implemented, and it improves treatment outcomes of uterine cervical cancer [7]. However, no rule or consensus has been reached regarding the size or shape of high-risk clinical target volume (HR-CTV) or gross tumor volume (GTV) for actual indication of interstitial technique. We previously performed a simulation analysis using various sizes of HR-CTV models (at brachytherapy) with different volumes for tumor models to clarify the indication for each brachytherapeutic modality (ICBT, ICIS-BT, and interstitial brachytherapy [ISBT]). We found that an HR-CTV with size of 4 × 3 × 3 cm (36 cm3 in cube volume, 30 cm3 in ellipsoid) seems to be a threshold volume [8]. Therefore, for HR-CTV > 30 cm3, ICIS-BT or ISBT can be a better choice than ICBT (Figure 1). To translate these results into clinical situation, we assessed initial GTV to predict GTV and HR-CTV at brachytherapy using actual clinical patient data. Therefore, the aim of this study was to propose the size criteria for indication of interstitial technique.
Material and methods
Data from 112 patients (median age, 64 years; range, 24-86 years) with locally advanced cervical cancer treated at Kyoto Prefectural University of Medicine Hospital and National Hospital Organization Osaka Medical Center between July 1, 2014 and December 31, 2017 were included in the analysis. Table 1 shows patient and tumor characteristics.
Table 1
Patients characteristics
| Variables | Strata | No. or median (range) n = 112 | % |
|---|---|---|---|
| Age (years) | 64 (24-86) | ||
| Histology | Squamous cell carcinoma | 103 | 92 |
| Other | 9 | 8 | |
| FIGO stage | I | 2 | 2 |
| II | 28 | 25 | |
| III | 58 | 52 | |
| IVA | 13 | 12 | |
| IVB | 11 | 10 |
This retrospective study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine (approval No., ERB-C-776-3) and National Hospital Organization Osaka Medical Center according to ethical standards in the Declaration of Helsinki. Written informed consent was obtained from all participants included in the study.
Chemotherapy and external beam radiation therapy
All patients received EBRT. Computed tomography (CT) and magnetic resonance (MRI) images were fused, and GTV contours were created based on MRI. Kyoto Prefectural University of Medicine used an Aquilion 16 LB CT scanner (Toshiba Medical Systems Corporation, Otawara, Japan) for CT, and a 1.5 T Philips MRI scanner Gyroscan Intera CV Nova Dual system (Philips Medical Systems, Best, The Netherlands) for MRI. National Hospital Organization Osaka Medical Center used an Optima CT580 CT scanner (GE Healthcare, Milwaukee, WI, USA) for CT, and a 1.5 T Philips MRI scanner Gyroscan NT Intera and 1.5 T Philips MRI scanner Gyroscan Achiva Nova (Philips Medical Systems, Best, The Netherlands) for MRI. GTV, a macroscopic tumor, was defined as a region of high signal intensity on T2-weighted MRI images at diagnosis. Organs at risk (OARs) were created on CT. EBRT was performed in combination with cisplatin (40 mg per square meter of body surface area, once a week, for up to six doses). It was delivered as conventionally fractionated three-dimensional conformal EBRT with a four-field box technique, using a 10 MV photon beams with a linear accelerator. Treatment plan was based on CT images with 2.0 or 2.5 mm slice intervals. Initially, 30-40 Gy was delivered to the entire pelvis, followed by application of an antero-posterior and postero-anterior central shield through a 4 cm-width block to reduce the dose to the rectum and bladder, until total prescription dose for the pelvic side wall of 50 Gy was reached. A boost for lymph node metastasis was an additional 6-10 Gy. Single-fraction doses for larger treatment field, including pelvis and para-aortic lymph node were 2 Gy and 1.8 Gy, respectively. Details of treatment schedule at each hospital are shown in Table 2. Treatment planning device for Kyoto Prefectural University of Medicine Hospital was Monaco v. 5.11 (Elekta, Stockholm, Sweden), and treatment planning device for National Hospital Organization Osaka Medical Center was Eclipse v. 10.0 (Varian Medical Systems, Inc., Palo Alto, CA, USA).
Table 2
Major external beam radiotherapy (EBRT) treatment schedules used at our institutions
Brachytherapy
Brachytherapy was performed in weekly sessions after 30-40 Gy of chemoradiotherapy. Single ICBT fraction dose ranged from 5.0 to 6.0 Gy, and median total prescribed dose was 19.5 Gy/3 fractions (range, 15-26 Gy). Single ICIS-BT fraction dose ranged from 6.0 to 6.5 Gy, and median total prescribed dose was 19.5 Gy/3 fractions (range, 18-26 Gy). Single ISBT fractional dose ranged from 5.0 to 6.0 Gy, and median total prescribed dose was 25.0 Gy/5 fractions (range, 25-30 Gy). ISBT patients received the full dose from a single applicator insertion in five divided doses, twice a day, for three consecutive days, with at least six hours between doses. Treatment plan was based on a CT scan taken before first treatment fraction. After insertion of applicator in each session, CT and MRI images with a slice thickness of 3 mm were acquired using a large bore CT simulator and a 1.5 T diagnostic MRI system [9]. MR images were fused to create a contour, and HR-CTV was defined based on MRI [8, 9]. HR-CTV included residual lesions and the entire cervix, and treatment planning was performed using Oncentra®Brachy (Nucletron-Elekta, Stockholm, Sweden). All treatments were performed with a high-dose-rate (HDR) remote afterloading system (MicroSelectron® HDR, Nucletron-Elekta, Stockholm, Sweden). To optimize ICBT and ICIS-BT, prescription dose was administered at point A, and source intensity was determined using Manchester method. Then, after graphical optimization and manual adjustment of the dose distribution, ratio was modified based on HR-CTV coverage to determine the final prescription dose. ISBT optimization was first planned by geometrical optimization and modification of the dose distribution curve by graphical optimization and manual adjustment. After the correction of isodose curve was completed, the final prescription dose was determined and its percentage was modified according to HR-CTV coverage. The goal was to maintain the dose to OARs as low as possible while keeping D90 of HR-CTV (dose covering more than 90% of HR-CTV) above the planned prescribed dose.
Dose calculation and dosimetry indices
In summing the dose of EBRT and brachytherapy, the equivalent dose in 2 Gy fractions (EQD2) according to linear quadratic model was calculated as follows:
where N is the number of fractions, and d is the dose per fraction. To calculate tumor and OARs doses, α/β was assumed as 10 Gy and 3 Gy, respectively. Dose contribution from central shield was not considered. Minimum dose covering 90% of HR-CTV (HR-CTV D90) in EQD2 was used as the representative dose of brachytherapy [10].
Statistical analysis
All statistical analyses were performed using StatView v. 5.0 software (SAS Institute Inc., Cary, NC, USA). Student’s t-test was applied for normally distributed data, and Mann-Whitney U-test for skewed data. Chi-square test was conducted to analyze percentages. P < 0.05 was considered statistically significant.
Results
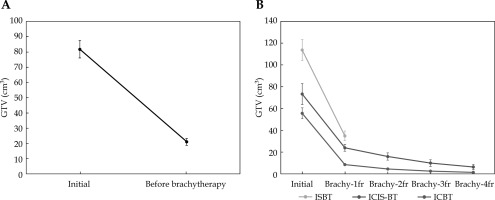
Table 3 shows a comparison of the three groups. ISBT treated advanced diseases compared with ICIS-BT and ICBT. The average GTV was 81.7 cm3 (range, 4.4-343.2 cm3) at diagnosis, which shrank to 21.0 cm3 (range, 0.0-124.8 cm3). The GTV shrinkage ratio was 25.8% (Figure 2A). Those were from 55.5 cm3 (range, 4.4-140.5 cm3) to 8.6 cm3 (range, 0.0-25.3 cm3) in ICBT (shrinkage ratio: 15.5%), from 113.7 cm3 (range, 31.4-343.2 cm3) to 34.9 cm3 (range, 0.9-124.8 cm3) in ISBT (30.7%), and from 69.8 cm3 (range, 28.2-107.4 cm3) to 24.3 cm3 (range, 12.3-40.1 cm3) in ICIS (34.8%) (Figure 2B).
Table 3
Comparison among three groups
Fig. 2
Changes in gross tumor volume (GTV). A) GTV shrinkage between initial GTV and before brachytherapy. B) Volume change of GTV between initial GTV and GTV at each brachytherapy among three techniques. Values are shown in average ± SE. Interstitial brachytherapy (ISBT) patients received full dose from a single applicator insertion in five divided doses, twice a day, for three consecutive days, with at least six hours between doses. Treatment plan was based on a CT scan taken before first treatment fraction
EQD2 for each treatment technique is depicted in Figure 3. For HR-CTV, an ISBT dose of 89.10 Gy was pre-scribed in EQD2 (range, 65.5-107.6 Gy), which was higher than those of ICIS (75.3 Gy; range, 63.5-79.7 Gy) and ICBT (75.1 Gy; range, 48.8-95.5 Gy) (p < 0.0001, Figure 3A). For bladder D2cm3, an ISBT dose of 86.9 Gy was prescribed in EQD2 (range, 58.0-182.2 Gy), which was higher than those of ICIS (72.3 Gy; range, 58.4-94.3 Gy) and ICBT (73.9 Gy; range, 38.0-117.3 Gy) (p < 0.0001, Figure 3B). For rectum D2cm3, an ISBT dose of 78.4 Gy was prescribed in EQD2 (range, 54.1-111.9 Gy), which was higher than those of ICIS (57.4 Gy; range, 46.4-67.3 Gy) and ICBT (55.5 Gy; range, 37.8-79.4 Gy) (p < 0.0001, Figure 3C). The EQD2 dose of the bladder and rectum included EBRT dose.
Fig. 3
Equivalent dose in 2 Gy fractions (EQD2) used in each technique. A) HR-CTV D90 + WP (EQD2), B) bladder D2cm3 (EQD2), C) rectum D2cm3 (EQD2)
HR-CTV – high-risk clinical target volume, WP – whole pelvis, ICBT – intra-cavitary brachytherapy, ICIS-BT – intra-cavitary and interstitial brachytherapy technique, ISBT – interstitial brachytherapy
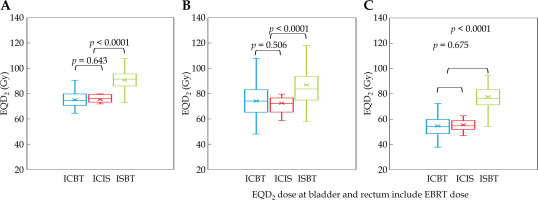
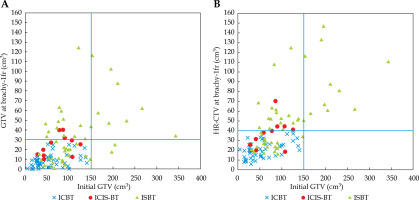
Figure 4A and B shows the correlation between initial GTV and GTV at brachytherapy and HR-CTV. We used interstitial technique for GTV at brachytherapy > 30 cm3 and HR-CTV > 40 cm3. In addition, all tumors with GTV > 150 cm3 received ISBT.
Discussion
In this study, we present actual clinical patient data to predict the indication for each brachytherapeutic modality, especially for individuals who needed interstitial technique that would be beneficial in creating an ideal decision tree to prepare a treatment system. ISBT with perineal approach is a useful treatment modality for advanced uterine cervical cancer [3, 4, 11, 12]. Multiple applicators can be implanted in and/or around CTVs freely, which allow for better CTV coverage compared with ICBT. Hsu et al. reported better coverage of ISBT for paracervical and parametrial extensions compared with ICBT using simulation analysis [13]. The guidelines of the American Brachytherapy Society for HDR brachytherapy for cervical carcinoma recommend that the eligibility criteria for undergoing ISBT include bulky lesions, narrow vagina, inability to enter cervical os, lateral extension, and lower vaginal extension [14, 15].
Recently, plastic or titanium interstitial catheters have been developed along with non-metallic template guidance techniques instead of stainless interstitial catheters and/or template contained metallic materials, which have made MRI use difficult. Interstitial technique from perineal skin could widen its application with the aid of three-dimensional image guidance, including MRI. However, indication for interstitial needles regarding the size or shape has not been established, and decision depend on the experience of physician and tumor response at initial brachytherapy.
We have previously analyzed tumor volume to elucidate the indication for each brachytherapeutic modality [8]. We found that an HR-CTV with size of 4 × 3 × 3 cm (rectangular volume, 36 cm3) seemed to be a threshold volume in a previous simulation analysis [8]. In a larger HR-CTV, ICIS-BT or ISBT is a better choice. In other words, we can hypothesize that a distance < 2 cm between the HR-CTV margin and the applicator axis (HR-CTV diameter < 4 cm if the applicator is located in the central area) represents a threshold for application of interstitial technique (Figure 1), which is calculated as 33 cm3 (approximately 30 cm3) in a sphere volume (HR-CTV diameter, 4 cm). Furthermore, if the distance between the HR-CTV margin and the applicator tip increase to 2.5-3 cm (HR-CTV diameter < 5-6 cm, HR-CTV volume of 65-113 cm3, approximately < 60 cm3), we speculated that ISBT is feasible to obtain adequate dose to tumors approximately 60 cm3 or more (Figure 1).
As the initial tumor volume reduced to 25.8% (74.2% reduction) after external beam radiotherapy with concurrent chemotherapy, we can suppose that an initial GTV volume of 116 cm3 could reduce to 30 cm3 at brachytherapy. We can subsequently recommend preparation for interstitial technique in cases of initial GTV of 116 cm3 or more, and also recommend multicatheter interstitial brachytherapy for an initial GTV of 233 cm3 or more (which may reduce to 60 cm3) (Figure 1). An initial GTV of 150 cm3 can be estimated as a threshold value required for interstitial technique based on our experience (Figure 4A, B).
Our data are in line with those in previously published studies [16-20]. The Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline [21] has recently recommended that D90 ≥ 85 Gy is reasonable for patients with poor response or large volume (tumor diameter > 4 cm) disease. It was stated that the utilization of a hybrid intra-cavitary/interstitial technique can help improve dose distribution when appropriate target and/or OARs dose constraints with an intra-cavitary alone approach are not achieved. Furthermore, a study conducted in India reported that a tumor volume ≥ 70 cm3 is a poor local control factor [22], which indicates the limitation of ICIS-BT and requirement of sole interstitial ISBT. Sometimes, ovoid and tandem themselves are obstacles for inserting interstitial needles.
Lateral tumor extension can be controlled with ICIS-BT. However, vertical extensions, such as uterine corpus direction, are difficult to be covered by ICIS-BT (prohibiting adequate tandem position), and are frequently observed in patients with local disease relapse [15]. In this regard, corpus invasion results in an irregular shape of the uterine corpus, which is an additional risk factor for requirement of intra-corpus interstitial catheters to adequately cover extensive tumor extent.
This was a retrospective study conducted at a few institutions with a limited number of patients. Further investigations are warranted to verify the findings demonstrated in the current analysis.