Purpose
Endometrial cancer (EC) is the most common gynecological cancer among post-menopausal women in developed countries [1, 2]. The standard of care for primary treatment includes hysterectomy plus bilateral salpingo-oophorectomy with or without pelvic and para-aortic lymph node dissection. Vaginal cuff has been demonstrated as the main location of relapses after surgery, and post-operative radiotherapy (RT) has been proven to reduce the local relapse rate after surgery [3]. Therefore, adjuvant RT is recommended for patients with high-risk clinical and pathologic features, such as age > 60 years, high pathologic grade, aggressive histology, ≥ 50% myometrial invasion (MI), lympho-vascular space invasion (LVSI), non-endometrioid tumor histology, lymph node metastases, and tumor extension into cervix or vagina [4-10]. In PORTEC-2 trial [11], patients were randomized with respect to post-operative RT techniques, such as external beam RT (EBRT) or vaginal cuff brachytherapy (VC-BRT), and demonstrated a similar rate of reduction in relapses for both techniques, but VC-BRT showed significantly less gastrointestinal and genitourinary toxicities. Therefore, VC-BRT without EBRT is becoming a standard adjuvant treatment approach in selected patient groups. However, the type of VC-BRT technique depends on the thickness and previous or possible extension of the tumor within the vagina. Single-channel BRT (SC-BRT) cylinder is the most commonly used intra-cavitary applicator for VC-BRT. However, it is limited in its ability to sculpt dose distribution around target volumes and critical structures (e.g., the rectum, bladder, small bowel, etc.) due to single-channel anisotropy. On the contrary, multi-channel BRT (MC-BRT) cylinder with additional channels added along the periphery of applicator, allows users to generate asymmetric dose distribution, not possible with SC-BRT applicator. Nevertheless, both of these approaches are generally suitable for patients presenting non-bulky disease with a depth or thickness ≤ 5 mm, and interstitial BRT (or intra-cavitary plus interstitial BRT) is recommended for patients with bulky residual disease after surgery or asymmetric tumor extension with a depth > 5 mm [12-16]. However, interstitial BRT has some limitations, such as requiring general anesthesia, hospitalization, and additional experience in the field. Recently, we introduced a novel intensity-modulated BRT (IM-BRT) applicator that is a combined version of MC-BRT applicator with directional compensator materials, including aluminum, titanium, stainless steel, and cerrobend alloy, for dose modulation, to bridge the gap between the tolerability of MC applicator and dosimetric advantages of interstitial brachytherapy in selected patient groups [17]. In the present study, a prospective evaluation of the dosimetric performance of IM-BRT applicator compared with SC-BRT and MC-BRT applicators for high-dose rate (HDR) VC-BRT was presented.
Material and methods
Patient election
This dosimetric study was approved by the Institutional Clinical Researches Ethics Boards of Hacettepe University (approval No.: KA-2373). All patients were informed about the nature of the procedure and possible side effects before each application. In total, 15 patients with uterine-confined EC, who were treated between August 2021 and February 2022 at Hacettepe University, Department of Radiation Oncology, were included in the current study. All patients were diagnosed with intermediate-risk disease, including stage IB, grade 1-2, endometrioid type EC without abundant LVSI after surgery, and received adjuvant high-dose-rate VC-BRT only. During gynecological examination, it was ensured that the patient vaginal vault healing was completed, and vaginal anatomy was suitable for in-house IM-BRT applicator, which details have been described previously [17]. Each patient underwent CT scan with commercially available SC applicator, diameter of 35 mm (CT- and MR-compatible vaginal cylinder, GM11004140; Varian Medical Systems, Inc., Palo Alto, CA, USA) using Toshiba Aquilion LB CT Simulator (Toshiba Medical Systems, Otawara, Japan); all patients were treated with standard clinical protocol using SC-BRT. After standard CT scanning process, IM-BRT applicator with a diameter of 35 mm was inserted, and second CT scan was performed for dosimetric comparison purpose only. Before CT simulation, all patients were requested to empty their bladder and rectum. As a scanning protocol, 100-120 kVp tube voltage, 300-350 mAs current value, and 2.5 mm slice thickness were applied. After simulation processes, CT images were transferred to BrachyVision Acuros treatment planning system (TPS), version 13.5 (Varian Medical Systems, Palo Alto, CA, USA) for contouring and treatment planning processes.
Contouring and treatment planning
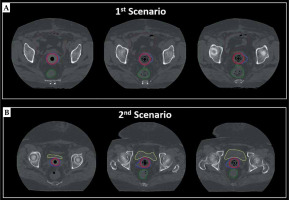
CT data sets, which were scanned for research purposes, were used to generate 3-dimensional (3D) treatment plans. In terms of clinical target volume (CTV) definition, two different scenarios were simulated to evaluate the dosimetric performance of IM-BRT applicator. In the first scenario, standard CTV, called CTVs, were defined as the proximal 3.5 cm of the vagina, including the entire thickness of vaginal wall via CT guidance, which is the standard target volume definition used in our routine clinical practice. In the second scenario, virtual CTV, called as CTVv, were defined by adding extra lateral extension to the reference CTVs to simulate the tumor with a thickness > 5 mm, ranging between 7 mm to 25 mm, as illustrated in Figure 1. The bladder, rectum, sigmoid colon, and small bowel were also delineated as organs at risk (OARs) [18].
Fig. 1
Typical view of delineated CTVs (red) and CTVv (blue) volumes on axial CT images of (A) first scenario and (B) second Scenario
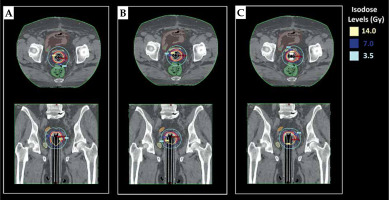
Three different treatment plans were generated for two different CTV: 1. SC-BRT treatment plans for CTVs and CTVv with centrally located rigid guide tube; 2. MC-BRT treatment plans for CTVs and CTVv with one central rigid guide tube and eight peripheral catheters; and 3. IM-BRT treatment plans for CTVs and CTVv with one central rigid guide tube, 4-6 peripheral catheters according to patient anatomy, and 1-2 compensator materials (e.g., aluminum, titanium, and stainless steel) in anterior and/or posterior direction to spare the rectum and/or bladder as much as possible. During treatment planning, same CT dataset that was scanned with IM-BRT applicator was applied for all approaches, including SC-BRT, MC-BRT, and IM-BRT plans, to eliminate all uncertainties due to differences in application and patient anatomy. A prescription dose was set as 7 Gy per fraction, for a total of 21 Gy. During treatment planning, volume optimization tool was used to find an optimal solution, and similar input parameters (Table 1) were defined to make it user-independent for inverse planning technique. Initially, all treatment plans were calculated based on the TG-43 formalism as a standard step in defined version of TPS. Then, Acuros®BV (grid-based Boltzmann equation solver) calculation algorithm available in Varian BrachyVision TPS was applied for heterogeneity correction for all plans. After final dose calculation, normalization was performed, such that 95% of CTVs and CTVv received at least 100% of the prescribed dose for all plans.
Table 1
Optimization parameters for volume optimization
Plan evaluation
During plan evaluation, dose volume histogram (DVH) parameters of CTVs and CTVv (D98, D95, D50, and D2), bladder (D2cc, V5Gy, and V7Gy), rectum (D2cc, V5Gy, and V7Gy), sigmoid (D2cc, V5Gy, and V7Gy), and small bowel (D2cc, V5Gy, and V7Gy) were noted for a dosimetric comparison of SC-BRT, MC-BRT, and IM-BRT plans. Moreover, the total volume receiving dose equal or greater than 200% of the prescription dose in external body (V200) was reported to evaluate higher dose volumes for all applicator geometries, which may be important for necrosis. Calculated total dwell time in 370 GBq source activity per fraction was also analyzed to evaluate total irradiation time during treatment.
Statistical analysis
All data were recorded and analyzed with Statistical Package for Social Sciences (SPSS) software (version 20, IBM). Descriptive statistics (mean and standard deviation) were calculated, and unpaired Student’s t test was used to assess the relationship between treatment planning parameters of the three different approaches, such as SC-BRT, MC-BRT, and IM-BRT. P-value < 0.05 was considered statistically significant.
Results
Data in Tables 2 and 3 present the quantitative dosimetric comparison of SC-BRT, MC-BRT, and IM-BRT plans created for two different scenarios, including CTVs and CTVv, respectively. The mean CTVs volume was 13.3 cc (range, 9.1-25.9 cc), and the mean CTVv volume was 15.2 cc (range, 10.5-31.3 cc). For the first scenario, the ranges of the mean values for D98, D50, and V200 were within 674.8-676.1 cGy, 843.2-848.1 cGy, and 0.2 cc for all approaches, respectively. However, there was a statistical difference (p = 0.025) regarding D2 value of CTVs between IM-BRT and SC-BRT. In terms of OARs’ sparing, the rectum (V7Gy) and bladder (D2cc and V5Gy) values were all found to be significantly lower in IM-BRT than in SC-BRT (p < 0.036) plans. For the second scenario, IM-BRT provided equivalent D98 value with smaller deviation compared with MC-BRT. However, both IM-BRT and MC-BRT were found to be significantly better than SC-BRT regarding D98 value of CTVv. In terms of OARs sparing, IM-BRT was observed to be significantly better than SC-BRT for the rectum (D2cc, V5Gy, and V7Gy), bladder (D2cc and V7Gy), and small bowel (D2cc, V5Gy, and V7Gy) values. On the other hand, DVH parameters of the sigmoid showed large difference between IM-BRT and SC-BRT plans, but these were not statistically significant, with p-values for D2cc, V5Gy, and V7Gy as 0.09, 0.12, and 0.06, respectively. Similarly, although there were no statistical differences between IM-BRT and MC-BRT in terms of OARs sparing, except for the rectum V7Gy, the mean value of all defined DVH parameters for OARs were found considerably lower in IM-BRT plans than in MC-BRT plans. As illustrated in treatment plans created for a representative case, IM-BRT applicator provided more conformal dose distribution, especially for CTVv, which was not possible with standard MC applicator (Figure 2). In addition to DVH parameters, the total mean dwell time in 370 GBq source activity for SC-BRT, MC-BRT, and IM-BRT plans were found as 317.0 s, 297.8 s, and 330.1 s for CTVs, and 377.8 s, 329.6 s, and 364.6 s for CTVv, respectively (Table 4).
Table 2
Comparison of DVH parameters (CTVs) for SC-BRT, MC-BRT, and IM-BRT plans
Table 3
Comparison of DVH parameters (CTVv) for SC-BRT, MC-BRT, and IM-BRT plans
Discussion
The current paper introduced the dosimetric performance of a novel IM-BRT applicator for adjuvant HDR VC-BRT. Standard MC applicators generally lose their advantage in patients with bulky residual disease with approximately > 5 mm thickness, and interstitial BRT is frequently required [15, 16, 19, 20]. Although, interstitial BRT provides better dose distribution in selected patient groups, it needs an expertise in the field, general anesthesia, and hospitalization. As stated by Govindaraj et al. [21], there is about 7% risk of wound infections, abscesses, and fat necrosis after interstitial BRT application. All these parameters make interstitial technique challenging in routine clinical practice; therefore, IM-BRT is gaining popularity to create more conformal treatment plans, which may be alternative to interstitial BRT plans [22, 23]. Here, we proposed a novel IM-BRT applicator that might be useful in selected patient groups. The special design of IM-BRT applicator allows for using different combinations of the source transfer channels as well as desired type and lengths of directional compensator materials, which were discussed in our previous study [17]. The main purpose of the directional compensator is not to provide complete shielding of OARs, but to modulate dose distribution according to patient anatomy. Our data revealed that IM-BRT plans had consistently lower OARs doses compared with both SC-BRT and MC-BRT plans, without compromising the target coverage. The advantage of IM-BRT applicator was most apparent for the second scenario, in which the treatment plans were created for CTVv. For this scenario, the mean value of rectum D2cc for IM-BRT was almost 14% (p < 0.01), and 8% (p = 0.08) lower than for SC-BRT and MC-BRT plans, respectively. Similarly, a noticeable dose reduction in the mean value of bladder D2cc was achieved with IM-BRT, which was almost 14% and 4% lower than in SC-BRT (p = 0.02) and MC-BRT plans (p = 0.22), respectively. However, we need to emphasize that D2 of CTVv increases with the use of MC-BRT (7.8%, p = 0.12) and IM-BRT applicators (15.7%, p = 0.03) compared with SC-BRT. Similar to our study, Bahadur et al. [24] reported that MC-BRT applicator caused 15% (p = 0.0002) increase in vaginal mucosa dose, as estimated by D2 of CTV compared with SC-BRT applicator. The reason was directly associated with the fact that since the peripheral source transfer channels in MC-BRT applicator were placed close to the vaginal mucosa, the dose gradient in the radial direction was very steep and more heterogeneous than in SC-BRT plans [24]. Therefore, extra attention must be paid for vaginal mucosa dose, especially in case of CTV extension > 5 mm, to keep under tolerance levels, as recommended by ABS guidelines [25]. Another disadvantage of MC-BRT applicator is that the total planning time (although varies based on experience of the planner) may be required longer, with about 1.5-2.0 fold longer compared with SC-BRT plans due to several parameters, including reconstruction of multiple catheters, controlling the loading patterns of each catheter after optimization process, etc. Additionally, IM-BRT plans require extra time for pre-planning procedure, with the settings of compensator materials. Govindaraj et al. [21] stated that MC-BRT generally require longer planning time, and the mean value for total planning time was 120 min, with SC-BRT planning time as expected. In contrast to the total planning time, the mean of total dwell time for MC-BRT was significantly lower than for SC-BRT plans (p = 0.02 for CTVs, and p = 0.01 for CTVv). The reason of this decrease can be that peripheral channels in MC-BRT require less dwell time to deliver same radiation dose to the defined depth due to an inverse square law. However, compensator materials used in IM-BRT applicator cause a statistically significant increase (p < 0.02 for CTVs, and p = 0.01 for CTVv), around 11% in the total dwell time compared with MC-BRT due to extra attenuation of the compensator materials. Nevertheless, this is a reasonable increase, which is about 32 s increase over 5 min. per fraction for 370 GBq source activity. Moreover, the mean of total dwell time for IM-BRT was found to be comparable with SC-BRT applicator, even 3.6% lower for CTVv plans. However, the type and geometric design of the compensator or shielding material may significantly affect the total dwell time ratio over standard SC or MC applicators geometry. In the literature, similar to our applicator, Skinner et al. [26] introduced another IM-BRT applicator for different shielding design types using high-Z materials, and they reported that dwell times for the shielding design were about 1.3 times longer than the 6-channel MC-BRT applicator for the equivalent fully symmetric dose distribution.
The present study has some limitations that need to be recognized. The first one is that, since this study was prospective dosimetric study in nature among volunteer patients, we were not able to perform more than one CT scanning due to an extra radiation dose. Therefore, all CT scans with IM-BRT applicator were performed only for pre-planning, as defined in our previous study [17], which was a CT scanning without compensator materials. The type, length, and position of compensator materials were defined according to patient anatomy using an automated contouring template, and all treatment plans were created according to virtually created compensator materials. Therefore, we could not verify it with second CT scanning. Nevertheless, all these conditions were confirmed with a 3D-printed quality control phantom, which was presented in our previous work [17]. Another limitation, also stated by Skinner et al. [26], was the highest density material available in the current version of Acuros BrachyVision calculation algorithm limited to stainless steel (Acuros material table version 13.5), with a maximum density of 8 g/cm3. Therefore, the possible advantage of cerrobend alloy in suitable patients, especially for CTVv plans, could not be simulated in the present study. Finally, this study focused only on the dosimetric comparison of IM-BRT plans with SC-BRT and MC-BRT plans. However, as also stated by Bahadur et al. [24], large intra-fractional dose variation may occur due to the marked difference in the rectum and bladder volume during using MC-BRT in inverse planning VC-BRT. Additionally, in contrast to SC-BRT, intra- and inter-fractional differences in applicator rotation, especially for IM-BRT plans, may cause significant differences in dose distributions. Therefore, all these parameters need to be further investigated.
Conclusions
While the IM-BRT applicator is still in a pre-clinical phase, our investigation demonstrated the proof-of-concept in real patient treatment plans with promising dosimetric results compared with MC-BRT. IM-BRT may possibly allow for dose escalation in circumstances, in which it can be beneficial or used as an alternative to interstitial BRT for VC-BRT in selected patient groups.