Summary
In our study we analyzed possible factors that may contribute to severe pain during percutaneous coronary intervention. We found that masculine gender, greater weight and height as well as diabetes mellitus and myocardial infarction diagnosis on admission correlated with lower pain level. Conversely, greater maximal and minimal diameters of the radial artery correlated with stronger pain level. Our results should lead to better identification of patients who are prone to more severe pain and intensified analgesic perioperative treatment for those patients.
Introduction
Radial artery interventions significantly reduced the number of vascular complications compared to femoral access [1]. In our study we analyzed discomfort related to the procedure in a population of patients without spasmolytics use, previously described by our group. To prevent this pain, subcutaneous lidocaine is usually used. Some operators routinely use systemic drugs, but in our department it is performed only with a subcutaneous anesthetic agent in the vast majority of cases. Nowadays, procedural time is decreasing with the fast evolution of current material devices and the improvement of their flexibility, size, and deliverability, and we assume that perception of discomfort or pain nowadays should be less, especially due to the development of coronary artery equipment.
However, limited data are available in medical databases. Most of the research work focuses on the premedication and staining of lidocaine before surgery [2–5] and not on factors that may be a predictor of pain in a patient during coronary angiography to the radial artery. We sought to determine which group of patients is more prone to pain during interventions, thus trying to select those that would need more intensive pain relief during the procedure (e.g. analgesic premedication).
Aim
The main aim of our study was to assess what clinical and periprocedural factors affect discomfort and pain intensity during angiography via the radial artery without routine use of spasmolytics.
Material and methods
Study group
A single-center retrospective study was conducted. We reanalyzed our population of 3980 patients, which has already been the subject of our manuscript that was published in Advances in Interventional Cardiology in 2021. The final number of patients included in the study was 238. The study did not need separate bioethical commission approval according to the regulatory document KNW/0022/KB/69/18. The study was conducted at the First Department of Cardiology of the Upper Silesian Medical Center and covered the period from October 2016 to January 2018. During this period all successive patients who underwent angiography through the radial artery without use of spasmolytics drugs were asked about pain sensations during the procedure. We used the NRS (Numerical Rating Scale) to assess pain intensity. Next, we grouped patients into three separate categories: patients with up to 3 points were treated as the low pain group; patients with 4–7 points as the moderate pain group; patients with 8–10 points as the severe pain group. The study was performed on adult patients. Exclusion criteria were: 1) femoral approach (e.g. patients who were switched to the femoral route during the procedure because of spasm or tortuosity), 2) usage of spasmolytic drugs, 3) presence of spasm during the procedure (pain assessment would not be objective in this group). The PRISMA flowchart reporting numbers of patients and exclusions is presented in Figure 1.
Radial access, procedure steps
The patients before the procedure had their pulse palpated on both radial arteries.
Treatments were performed through the radial arteries without premedication prior to the procedure. To numb the puncture site of the radial artery, the patient was given 2 ml of lidocaine by subcutaneous injection. The radial artery access was achieved by the use of open bore needles, 0.025″ hydrophilic short guidewires and 6 Fr or 5 Fr Balton vascular access sheaths. To lower the risk of obstruction of the radial artery, 5000 IU of unfractionated heparin was injected as a bolus immediately after insertion of the sheath.
In all procedures, operators used 0.035” guides for insertion of catheters and standard 5F or 6F catheters. The vascular sheath was removed immediately after the procedure, and to obtain full homeostasis of the access site, standard tourniquets were used; they were inflated with air and removed after the required amount of time.
Immediately after the procedure, the patient was asked about forearm pain and the general feeling of discomfort during the procedure on a scale from 1 to 10. Ten points represented the maximum pain intensity and one point corresponded to no, or minimal discomfort.
Periprocedural assessment
On the following day the radial and brachial arteries were assessed by ultrasound in order to assess their maximum and minimum diameter or to detect their occlusion. Each radial artery imaging was performed postoperatively, in cross-sectional imaging, by putting the probe 2 cm proximally to the styloid process of the radius perpendicular to the arterial wall, above the puncture site.
Statistical analysis
Patients from three distinct subgroups (low, moderate, severe pain) were analyzed. Basic descriptive statistics included: mean and standard deviation for continuous variables and percentage values for categorical variables. The Shapiro-Wilk normality test was used, followed by the nonparametric ANOVA Kruskal-Wallis test for continuous variables. For categorical variables, Pearson’s χ2 test was used when sample sizes were large, while for smaller sample sizes Fisher’s test was used. Additionally, Kendall’s tau correlation coefficient (continuous variables) and Cramér’s correlation coefficient V (categorical variables) were calculated. The significance level was set to p = 0.05. Statistical analysis was performed using the R programming language (version 4.3.2) with RStudio Integrated Development Environment [6].
Variables analyzed
We analyzed 38 characteristics of the patients in terms of the possible influence on the pain level during angiography. We analyzed the following variables: age (years), height (cm), weight (kg), body mass index (BMI) (kg/m²), estimated glomerular filtration rate (eGFR) (ml/min/1.73 m²), ejection fraction (EF) (%), fluoroscopy time (minutes), number of catheters (catheters used during the percutaneous coronary intervention (PCI) procedure (n)), brachial artery diameter (mm), maximal diameter of radial artery (mm), minimal diameter of radial artery (mm), diameter of vascular sheath (5F/6F/other), as well as binary variables: gender (male or female), procedure performed on right or left radial artery, number of vessels operated on during PCI (one/two or more), catheter type (Tiger (TIG)/other), catheter diameter (5F/6F), intravascular ultrasound (IVUS) examination conducted, fractional flow reserve (FFR) measurement conducted, pathological (> 0.9 mm) intima-media complex thickness (IMC), high origin of radial artery, atherosclerotic changes of radial artery, presence of hematoma, patency of the radial artery, diabetes, hypertension, peripheral arterial disease, hyperlipidemia, smoking, history of cerebral vascular accident (CVA), history of PCI, history of coronary artery bypass graft surgery (CABG), diagnosis on admission: arteriosclerosis, diagnosis on admission: myocardial infarction (MI), diagnosis on admission: heart defect (HD), diagnosis on admission: other. Table I presents continuous variables with means and standard deviations for all patients, as well as patients with low, moderate and severe pain. Table II presents categorical variables (as percentages) for all patients and patients with low, moderate and severe pain.
Table I
Characteristics of participants. Continuous variables
Table II
Characteristics of participants. Categorical variables
[i] Data are presented as percentages. CVA – cerebral vascular accident, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft surgery, MI – myocardial infarction, HD – heart defect, IVUS – intravascular ultrasound, FFR – fractional flow reserve, TIG – Tiger, IMT – intima-media complex thickness.
Results
The number of patients included in the study was 238. Most of the participants had a low pain level (n = 133 (55.88%)), while fewer participants were in the moderate pain level group and severe pain level group (n = 88 (36.97%) and n = 17 (7.14%), respectively).
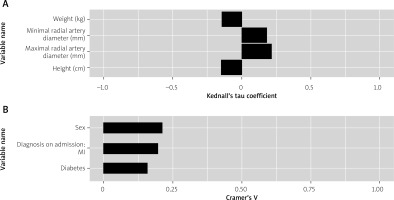
Aggregated analyses of the results are presented in Table III. Significant correlations are presented in Figure 2. Among 38 variables we found that only 7 had a significant impact on the pain level. Among those 7 variables 2 correlated with greater pain sensation and 5 correlated with lesser pain sensation. Greater weight (p = 0.027), greater height (p = 0.005) as well as masculine gender (p = 0.005), presence of diabetes (p = 0.045) and MI diagnosis on admission (p = 0.01) were associated with lower pain level. Greater maximal (p < 0.001) and minimal (p = 0.003) radial artery diameter were associated with increased pain level. Masculine gender was moderately associated with lesser pain (Cramér’s V = 0.212). Diabetes and MI diagnosis on admission were weakly correlated with lesser pain (Cramér’s V = 0.158 and 0.196, respectively). Similarly, greater height and weight were weakly correlated with lesser pain (Kendall’s tau coefficient = –0.147 and –0.141, respectively). In contrast, greater maximal and minimal radial artery diameter were weakly associated with increased pain level (Kendall’s tau coefficient = 0.215 and 0.18, respectively).
Table III
Results of correlation analysis
[i] SD – standard deviation, BMI – body mass index, eGFR – estimated glomerular filtration rate, EF – ejection fraction, CVA – cerebral vascular accident, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft surgery, MI – myocardial infarction, HD – heart defect, IVUS – intravascular ultrasound, FFR – fractional flow reserve, TIG – Tiger, IMT – intima-media complex thickness.
Discussion
We performed a single-centered retrospective study to assess which factors could possibly influence the pain level during coronary interventions via radical access. The data of 238 patients were analyzed. We found that greater weight, greater height, masculine gender, presence of diabetes and MI diagnosis on admission were associated with a lower pain level. Greater maximal and minimal radial artery diameter were associated with increased pain level.
Radial access for coronary interventions dramatically reduced complications compared to femoral access. Pain during and after examination can be caused by different factors including vascular problems, nerve damage and immediate site care (hemostasis) [7]. According to the authors’ knowledge there are only a few studies concentrating on pain during performance of cardiac interventions from the radial artery and the majority concentrate on post-procedural pain. We tried to find factors that could determine procedural pain, thus identifying those patients who would a priori require better, more efficacious periprocedural pain prophylaxis.
We concluded that a lower degree of pain during the procedure was observed in men. Similar observations were reported in other studies [8–13]. Bartley’s and Fillingim’s as well as Pieretti’s et al. reviews suggest that women are more prone to high pain sensitivity and risk for clinical pain. However, it is reported that many factors contribute to this phenomenon, e.g. genotype, endogenous opioid functioning, sex hormones and psychosocial mechanisms. The endogenous opioid system functioning revealed differences in women and men in pain-related activation. Men demonstrated larger magnitudes of mu-opioid system activation as the pain response in several brain regions [14]. Sex hormones and their receptors are associated with nociceptive transmission and may influence opioid pharmacokinetics and pharmacodynamics [15]. Estradiol and progesterone’s pain modulation influences are complexed and both exert pro-nociceptive and anti-nociceptive effects on pain, but testosterone appears to be more anti-nociceptive and its effects are stronger in men [16]. Psychosocial mechanisms include different pain coping strategies [17, 18], early-life exposure to stress and stereotypical gender roles – pain expression could be more socially acceptable among women – that may result in a biased reporting of pain in men [19].
We also concluded that the greater the height and weight of the patient are, the lower is the risk of severe pain. We are aware that these results may be strongly influenced by gender – there were statistically significant differences between mean weight for men and women and height. We also did not observe significant correlations between BMI and pain intensity. That was a surprising discovery for us, because there is evidence for impaired pain sensation in obese patients due to small fiber neuropathy [9]. In the National Health and Nutrition Examination Survey of people more than 40 years old, while 9% had peripheral neuropathy those who were diagnosed with obesity had a twofold higher risk (OR = 2.20, 95% CI: 1.43–3.39) of neuropathy compared to non-obese people [20]. It has also been proved that even non-diabetic obese patients show subclinical involvement of different diameter sensory fibers. Such impairment is related to insulin alterations and insulin resistance. Nevertheless, our study is not consistent with the study by Kilic, who found that a BMI > 29.8 kg/m2 predicted severe pain during coronary catheterization [12]. The authors supposed that the difficulty of reaching the artery due to adipose tissue may contribute to the problem. We did not observe this dependence.
Another discovery was that patients with DM are less likely to suffer increased pain during PCI via the radial artery. The most likely explanation in our opinion is diabetic neuropathy in this group of patients, as this condition results in a loss of sensory function beginning distally in the limb extremities, more commonly in the lower limbs, but findings of diabetic neuropathy indicate a loss of sensation in a ‘stocking and glove’ distribution as well [21].
MI diagnosis on admission was also correlated with lower pain levels. In our opinion this phenomenon is connected with rapid release of catecholamines, which have an antinociceptive effect. Norepinephrine is involved in pain modulation. It has been proven that sustained pain induces noradrenergic feedback inhibition of pain [22] as well as having an influence on the endogenous opioid system [23]. Unfortunately, it was not possible to analyze opioid usage preoperatively – information about the opioids administered by paramedics in the ambulance as well as after admission was not collected.
To our surprise, greater diameter of the radial artery was associated with a higher pain level. We could not find reports of other studies focusing on the relation between radial artery diameter and pain level. A possible explanation could be in the choice of vascular sheaths and risk of damaging the artery. Unfortunately, in our study we used measurements of radial and brachial arteries after the coronary intervention – the study did not include information about preoperative diameter of the arteries – one of the factors which influences the decision on the sheath size. We suggest that future studies could include measurements before and after an intervention with information about the vascular sheaths. This should provide further explanations regarding this freshly discovered phenomenon.
Important limitations of the study are the relatively small sample size, its retrospective character and acquiring data from a single center. The experience of the operator and number of puncture attempts were not assessed, yet they could be other potentially important factors. Another limitation is postoperative pain assessment – there is a possibility that memory of pain could be influenced by other factors such as time of the procedure [24]. A strong point of our study is that assessing possible factors influencing pain sensitivity during the PCI procedure is not widely described in the literature, e.g. in contrast to the post-procedural pain. This could be an interesting topic, as selecting potential subgroups of patients with an increased risk of intensified pain could result in better analgesic management.
Conclusions
In our study we analyzed possible factors which may contribute to severe pain experienced during PCI. We found that masculine gender, greater weight and height as well as DM and MI diagnosis on admission correlate with lower pain level. Conversely, greater maximal and minimal diameters of the radial artery correlated with stronger pain level. Our results should lead to better identification of patients who are prone to more severe pain and intensified analgesic perioperative treatment for those patients.