Antimicrobials are essential medicines needed for a basic healthcare system. However, developing antimicrobial resistance threatens the effective use of many antimicrobials, causing a severe global challenge [1, 2]. To overcome this, modern statistics and pharmacokinetics concepts are used to establish more directed dosing for a particular problem (given the patient and disease) [3]. Such a pharmacokinetic (PK) approach is included in the European Medicines Agency (EMA) guidelines for evaluating medicinal products, and it can also serve as a guideline for performing basic research involving phase IV clinical trials [4]. The spread of antibiotic resistance, as well as the insufficient knowledge about individualised, proper dosages in various patient populations, especially infants and children, and patients with organ failure, such as the intensive care unit population, prompts the search for rational and effective methods of optimising pharmacotherapy and selecting factors contributing to the increased resistance to treatment [3, 5]. In the case of anti-infective therapy, implementing PK principles makes it possible to determine the optimal dosing regimens of antimicrobials, which may lead to decreased bacterial resistance [6–8].
Validated PK models can be used to calculate antibiotic doses through Monte Carlo simulations that integrate pharmacokinetics, pharmacodynamics and microbiological data [9]. Simulation data enable us to predict the percentage of patients achieving PK/pharmacodynamic (PD) targets of antibiotic treatment for different dose regimes [10]. This type of Monte Carlo simulation is called the probability of target attainment (PTA). The cumulative fraction of response (CFR) method could be used to determine empirical therapy. CFR used expected population PTA values, data about microorganisms, and characteristic dosing schedules based on the population distribution of minimum inhibitory concentrations (MICs). It allows the probability of antibacterial therapy regimen success to be calculated in the absence of a specific MIC value [11]. This approach makes it possible to develop safe and effective antibiotic dosing, minimising the risk of subtherapeutic concentrations and consequently reducing the chance of inducing bacterial resistance [11, 12].
Bibliometric research provides a relatively quick, quantitative analysis of the available literature [13, 14]. It is a method used to examine medical lite-rature, allowing the identification of current trends and new directions in a particular area of medicine [14]. By analysing all publications fulfilling the inclusion criteria simultaneously, the bibliometric analysis provides a comprehensive view of an issue, making it more objective [13]. Despite its widespread use in the medical sciences, to our knowledge, this is one of the first, if not the first, bibliometric analyses focusing on the PK modelling of antibiotics.
Our study aimed to evaluate and summarise previous PK modelling studies of antibiotics conducted between 1983 and March 2023. Our comprehensive investigation, including authors’ scientific backgrounds, affiliations, countries, journals, keywords, co-citations, and individual publications, will allow future researchers to more easily identify the gap in previous research and notice the potential of PK modelling.
METHODS
Search methodology and database
We used the Web of Science (WoS) subscription from Clarivate Analytics to search for publications relating to antibiotic PK modelling in March 2023. We initially identified 1,276 relevant articles using this query: TS = ((“PK modeling” OR “PK modelling” OR “pharmacokinetic* modeling” OR “pharmacokinetic* modelling” OR “pharmacokinetic* model*” OR “PK model*” OR “population* pharmacokinetic*” OR “population* PK”) AND antibiotic*)
Then, using the available WoS filters, we narrowed the search to English-language articles, excluding proceeding papers, early access papers, and book chapters, identifying 968 publications from 1983 to March 2023. We detected no duplicates.
Data extraction
Due to the risk of retrospectively adding articles to WoS, we performed the data extraction process in one sitting. We obtained complete publication data with cited references. Given the restriction allowing up to 500 records to be downloaded simultaneously, we extracted two files, which we manually combined into one. According to the requirements of the respective programs, we chose the “plain text file” format for Bibliometrix (R library version 4.1.2, K-Synth Srl, University of Naples Federico II, Naples, Italy) [15] and CiteSpace (version 6. 1.R6, Chaomei Chen) [16], and the “tab delimited” format for VOSviewer (version 1.6.18, Nees Jan van Eck and Ludo Waltman, Leiden University, Leiden, the Netherlands) [17]. We used the Microsoft 365 Excel academic licence (Microsoft, Redmond, Washington, USA) to summarise the bibliometric data obtained.
Data analysis
We used Bibliometrix [15] and Microsoft 365 Excel to analyse and report: publication frequencies, country, organisation, journal, and author data, h-index, citations, and authors’ keyword occurrence. The country was attributed according to correspondence author affiliation. We used authors’ keywords, originally provided with the manuscript, to eliminate bias related to their automatic assignment by the WoS database. VOSviewer [17] allowed us to assess and visualise keyword co-occurrence (54 of the 1,410 keywords met the threshold set as at least ten keyword occurrences). In turn, we analysed reference co-citations using CiteSpace [16]. Using a scale factor equal to 25 and a timeframe from January 2000 to December 2022, the software qualified 870 records with a centrality of 925 nodes and 3,479 links.
RESULTS
Publications about antibiotic PK modelling
Following the inclusion and exclusion criteria, we selected 968 articles for bibliometric analysis. The citation count for all articles was 22,240 (20,115 without self-citations), while the average citation per publication was 22.98. The overall h-index was 65. Data about the ten top-cited articles are shown in Table 1.
TABLE 1
Most globally cited papers about antibiotic pharmacokinetic modelling
| Paper | DOI | TC | TC per year | Normalised TC |
|---|---|---|---|---|
| [32] Forrest A, et al., 1993, Antimicrob. Agents Chemother. | 10.1128/AAC.37.5.1073 | 899 | 29.00 | 7.29 |
| [39] Preston SL, et al., 1998, JAMA | 10.1001/jama.279.2.125 | 499 | 19.19 | 7.38 |
| [40] Gilks WR, et al., 1995, J. R. Stat. Soc. C-Appl. | 10.2307/2986138 | 368 | 12.69 | 2.82 |
| [30] Tsuji BT, et al., 2019, Pharmacotherapy | 10.1002/phar.2209 | 368 | 73.60 | 26.21 |
| [33] Goncalves-Pereira J, et al., 2011, Crit. Care | 10.1186/cc10441 | 255 | 19.62 | 5.44 |
| [41] Ambrose PG, et al., 2001, Antimicrob. Agents Chemother. | 10.1128/AAC.45.10.2793-2797.2001 | 246 | 10.70 | 2.99 |
| [26] Roberts JA, et al., 2009, J. Antimicrob. Chemother. | 10.1093/jac/dkp139 | 242 | 16.13 | 5.14 |
| [42] Mouton, JW, et al., 1994, Antimicrob. Agents Chemother. | 10.1128/AAC.38.5.931 | 230 | 7.67 | 3.58 |
| [34] Shekar K, et al., 2012, J. Crit. Care | 10.1016/j.jcrc.2012.02.013 | 188 | 15.67 | 3.62 |
| [43] Pai MP, et al., 2007, Pharmacotherapy | 10.1592/phco.27.8.1081 | 186 | 10.94 | 4.28 |
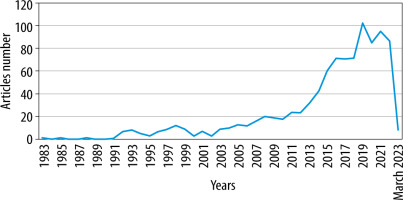
The articles analysed were published between 1983 and 2023, and the average annual growth rate, excluding the incomplete year 2023, was 35.56%. The three most productive years were 2019, 2021, and 2022, with 102, 95, and 87 papers, respectively. A detailed distribution is shown in Figure 1.
Publication countries
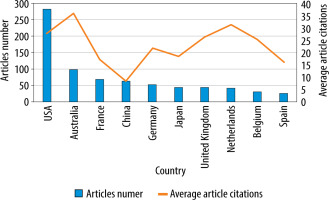
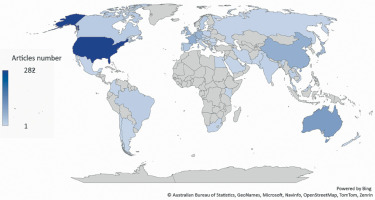
The United States of America (USA) was the most productive country, with 282 publications representing 29.13% of all identified articles. The second result was achieved by Australia, with 98 papers accounting for 10.12%. In third place was France, which published 68 articles, covering 7.02%. The most productive countries are shown in Figures 2 and 3.
Regarding the average number of citations, Portugal, Australia, and New Zealand scored the highest with 74.75, 35.94 and 34.00 per publication, respectively.
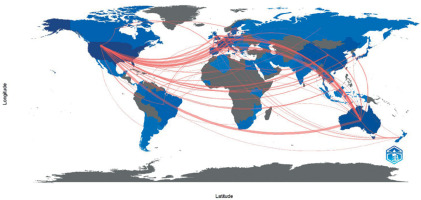
The USA research centres published most frequently in collaboration with other foreign centres (265 publications), among other countries. In second and third place were Australia and France, with 198 and 117 articles, respectively. The most extensive collaboration between the two countries was between the USA and Australia; they published 49 manuscripts in cooperation. This was followed by the Australian-French and USA-German collaborations, with 29 and 21 publications, respectively. A graphical representation of the observed partnerships is shown in Figure 4.
Publication journals
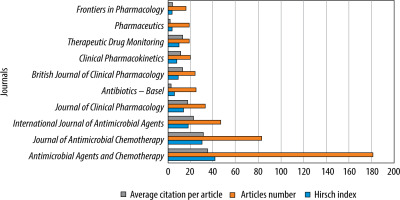
Out of 253 different journals, Antimicrobial Agents and Chemotherapy, with 181 published papers (18.70%), an impact factor of 5.938, and 9.3 CiteScore points, was the most productive journal, followed by the Journal of Antimicrobial Chemotherapy with 83 articles (8.57%), an impact factor of 5.758, and 9.4 CiteScore points, and International Journal of Anti-mi-crobial Agents with 47 manuscripts (4.86%), an impact factor of 15.441, and 16.1 CiteScore points. The top three journals identified collectively published almost one-third (32.13%) of all articles on antibiotic PK modelling. A summary of the involvement of the ten most productive journals is shown in Figure 5.
Publication authors
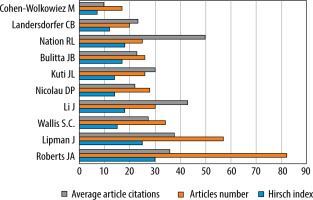
The ten most productive of the 4553 authors collectively published 345 of the 968 articles related to PK modelling of antibiotics, representing 35.64% of the papers (Figure 6). The author with the highest number of publications (82) was Roberts, who achieved an h-index of 30 and an average article citation count of 35.82. In second place was Lipman with 57 papers, an h-index of 25, and an average of 37.54 article citations, followed by Wallis with 34 papers, an h-index of 15, and 27.38 article citations on average.
Publications affiliations
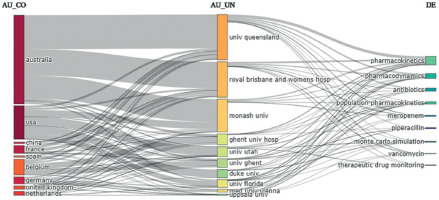
The University of Queensland in Australia achieved the highest number of affiliations in 159 publications on antibiotic PK modelling. The second result of 120 articles was obtained by the Royal Brisbane and Women’s Hospital, followed by Monash University with 116 papers. To illustrate the difference between the top three and the other units, the next two places were occupied by the Medical University of Vienna and the University of Florida, with an equal number of 37 articles. Additionally, we analysed the links between countries, affiliations, and keywords to present the impact of each institution on developing specific subfields in antibiotic PK modelling (Figure 7).
Co-cited references analysis
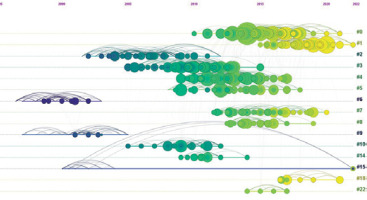
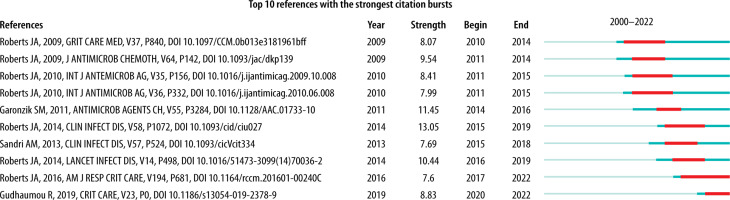
For the co-citation analysis, 870 references from 2000 to 2022 were identified and classified into 15 clusters according to keywords. The created timeline of the cluster map was characterised by a modularity (Q) of 0.8008, demonstrating a high degree of freedom in the distribution of the set elements. In turn, the high Silhouette score of 0.9112 (N = < –1; 1 >) represents the high consistency of the data present in the individual clusters. The newest clusters, #1, #18, #0, and #22, focused on target achievement, new antibiotic dosing, therapeutic drug monitoring, breakpoint, and central nervous system infection (Figure 8). The labels of the different clusters with their size, Silhouette score, average year, and different categorisations are shown in Table 2. Both sources (Figure 8 and Table 2) allow an assessment of the interest in the specific topic of PK modelling, including different antibiotics by year. The top 10 strongest citation bursts between 2000 and 2022 affecting the development of PK modelling of anti-biotics [8, 18-26] are shown in Figure 9.
TABLE 2
Summary of the most significant 15 clusters with top terms mentioned according to latent semantic indexing (LSI) and log-likelihood ratio (LLR)
Trends in antibiotic PK modelling
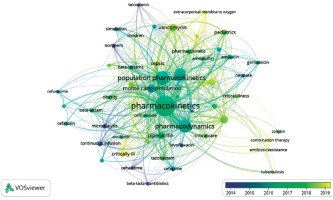
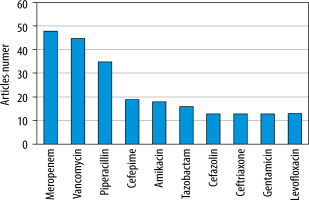
We used the authors’ keywords to analyse trends in the PK modelling of antibiotics. Out of a total of 1,410 keywords, we used 54 that reached at least ten repetitions to create the co-occurrence map. Analysing the size and colour of the dots presented allows one to assess interest in particular issues and antibiotics over the years (Figure 10). Among them, the top 5 keywords related to specific populations were paediatrics, critically ill, intensive care ex aequo with obesity, cystic fibrosis and critical care, with 23, 22, 19, 17 and 15 occurrences, respectively. Considering the most common antibiotics mentioned in the keywords, meropenem ranked first with 48 occurrences, followed by vancomycin and piperacillin with 45 and 35, respectively (Figure 11).
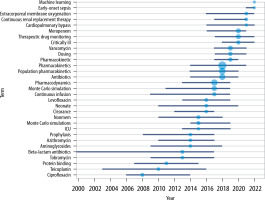
Using the authors’ keyword analysis, we explored the interest in using PK modelling of antibiotics to run Monte Carlo simulations, assess the need for monitored therapy, and reduce antibiotic resistance. These fields’ average annual growth rates were 66.76%, 59.11%, and 66.77%, respectively. The use of Monte Carlo simulation to develop the most effective antibiotic dosing regimens has started to gain popularity since 2006. Interestingly, machine learning was one of the most frequently occurring keywords in 2022. One population of particular interest in recent years has been critically ill patients (peak interest in 2020), with more in-depth research related to patients on extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT) a year later. The graphically presented keyword analysis also identifies the antibiotics with higher interest levels in particular years. A list of the top 3 keywords by year is shown in Figure 12.
DISCUSSION
Increasing bacterial resistance and the slow development of new antibiotics have led to a growing interest in individualising antibiotic therapy [27, 28]. One way to optimise pharmacotherapy is through population PK models and pharmacokinetic simulations to calculate potentially effective and safe dosing regimens [3, 29]. Therefore, the increased interest in using population modelling to limit bacterial resistance observed in our study should not be surprising. Beside analysing current trends in PK modelling of antibiotics, our bibliometric analysis identifies aspects to which the most attention has been given in research to date. Furthermore, based on the data presented, it is straightforward to indirectly identify areas that have not yet been sufficiently explored, represent a niche for future research or require further exploration.
Over 40 years, we have seen a constant growth in interest in PK modelling of antibiotics (the average annual growth rate is 35.56%). Our analysis shows that 968 articles have been published on this topic since 1983. The most rapid expansion of publications dedicated to antibiotic PK models began in 2012/2013, maintaining a steady increase until 2019. Given the continuing evolution of pharmacometrics [3], the limited potential for formulating new antibiotics, and increasing bacterial resistance [27, 28], we expect to observe a further rise in work aimed at the appropriate use of antimicrobial drugs. An indirect analysis of our findings identified antibiotic resistance, pharmacokinetic discrepancies between populations or treatment failure as some of the main reasons for the increased interest in PK modelling of antibiotics.
Taking into consideration the list of the ten most globally cited papers, and especially the high position of a relatively new article by Tsuji et al. [30], it can be seen that both scientists and clinicians reco-gnise the need to establish gold standards for antimicrobial drug dosing in different patient populations [3, 5, 31]. Furthermore, 4 of the 10 publications focused on PK and/or antibiotic dosing in critically/seriously ill patient populations [26, 32–34]. Particular interest in the intensive care population is due to the high inter-individual variability in PK parameters and dynamic changes in the patient’s condition, which require individualised and optimised drug dosing [35, 36].
The USA is a clear powerhouse regarding the number of published manuscripts and the collaborations undertaken with institutions abroad. Second in terms of productivity was Australia, which performed better than the USA in terms of the average number of citations per article (35.94 vs. 27.91). Among Asian countries, China and Japan were in the top 10. In comparison, France, Germany, the UK, the Netherlands, and Belgium achieved higher productivity, indicating a high interest in the topic among European countries. Leaving aside the USA with its strong lead, the level of interest in PK modelling of antibiotics among the top 10 is comparable. In turn, analysing the map of collaborations, one observes the spreading cooperation of institutions worldwide. However, the well-established networks are mainly between highly developed countries, whereas bacterial resistance is a global issue [1, 2]. Therefore, we believe new collaboration networks should be established in the future in which more countries, including developing countries, could participate.
As with the USA’s significant lead over other countries, the undisputed leader in the number of articles published has also been clarified for journals. In first place was Antimicrobial Agents and Chemotherapy, with 181 papers published, 2.18 and 3.85 times more than the Journal of Antimicrobial Chemotherapy and the International Journal of Antimicrobial Agents, ranked second and third, respectively. In contrast, the International Journal of Antimicrobial Agents is characterised by an almost 3-times higher impact factor (15.441 vs. 5.938 and 5.758) and a more than 50% higher CiteScore (16.1 vs. 9.3 and 9.4) compared to the first two journals in terms of productivity. In addition to journals focusing on antimicrobial drugs, the top 10 journals included those on general pharmaco-logy, clinical pharmacology, and pharmacy. This indicates a multi-disciplinary interest in the topic and facilitates the exchange of scientific information between different research communities.
According to our analysis, the author with the most publications was Roberts JA, who achieved the highest h-index value [30]. Roberts devoted his scientific work to antibiotic therapy research, making him one of the most characteristic figures in this field of medicine. Meanwhile, the highest average citation of articles among the top 10 productive authors was achieved by Nation, who ranked 8th in terms of the number of published papers.
Contrary to expectations, none of the first three places in terms of the number of published works was taken by an affiliate from the USA. Affiliations of scientific and research units from Australia occupied the entire podium. The Medical University of Vienna was in fourth place, followed by the University of Florida (USA). These results suggest significant dispersion between the individual institutions studying the pharmacokinetics of antibiotics in the USA and the specific interests of selected institutions in Australia.
Co-citation analysis identified 15 clusters cate-gorised by authors’ keywords. Thanks to the cluster-based timeline, we identified the most popular research areas in the context of antibiotic PK modelling. As expected, most attention was paid to using models for dosing calculations, developing therapeutic drug monitoring, and establishing new breakpoints for resistant pathogens. On the occasion of the co-citation analysis, we presented the ten strongest citation bursts. Considering the results of citation bursts, the above-average interest in publications on antibiotic PK modelling is about four years in the near interval from the date of publication.
Analysing trends using the authors’ keywords allowed us to identify the antibiotics that have received the most attention. As a group, β-lactams attracted the most significant interest, among which the largest number of studies focused on the pharmacokinetics of meropenem. The particular interest in meropenem is not very surprising, as it is one of the more commonly used antibiotics in the hospital setting, characterised by its broad spectrum of action and relatively high safety of use [26]. Furthermore, due to the reported high values of volume of distribution and clearance and the low protein-bound fraction, high variability of PK parameters is observed between different populations [33]. Also in the top 10 were vancomycin and amikacin. Both antibiotics have a narrow therapeutic index and high toxicity, so they are reserved for treating severe bacterial hospital infections [37]. In addition, prolonged treatment or high doses can lead to a sudden deterioration of kidney function, the main eliminatory organ [37, 38]. Therefore, monitoring renal clearance, which can decline due to progressive disease and the use of these antibiotics, is one of the main factors influencing possible dosage optimisation. Based on this analysis, future authors may identify antibiotics that may be potentially interesting, but their pharmacokinetics in different populations are still a knowledge gap. Moreover, using the keyword analysis, we confirmed the increase in interest in simulations aimed at dosing antibiotics that limit the development of bacterial resistance observed in the co-citation analysis. Increasing interest in combining PK modelling with simulation is becoming particularly important for populations with difficult-to-predict changes in patient condition, which is especially relevant for critically ill intensive care unit patients. This is indicated both by recent trends indicated using keyword analysis and published papers related to PK modelling in intensive care. Additionally, considering the most common keywords in recent years, we have observed a growing interest in implementing artificial intelligence (AI) and machine learning for PK modelling.
To maximise the accuracy of the analysis, we used three independent software applications, allowing us to use a variety of approaches to bibliometric analysis. We believe that our study’s results will be a useful starting point for researchers interested in modelling the PK of antibiotics. The study’s main limitation arising from the bibliometric analysis is its time-specific character associated with constantly updating the available literature. In addition, due to the risk of errors associated with incomplete data, we excluded processing papers, letters, books, and early-access articles, which may have led to omitting literature relevant to the topic under discussion.
CONCLUSIONS
The relative interest in antibiotic PK modelling has gradually grown over recent years. A significant increase in publications on this topic occurred in 2012/2013, maintaining an upward trend. Most studies have focused on meropenem, vancomycin, and piperacillin, suggesting more research opportunities for other antibiotics. The observed rising focus on population PK combined with Monte Carlo simulations and the probability of target attainment approach raises the potential for improving antibiotic dosing to reduce treatment inefficiencies and bacterial resistance progression. Nevertheless, the need for gold standards for antibiotic use in specific patient populations remains a challenge that requires further research. Future studies should focus on populations and antibiotics that are less frequently chosen or so far omitted from consideration. In turn, the increase of multi-centre international collaboration at the model building and sample collection stage is a great opportunity to build a model based on a larger number of varied patients, thereby minimising model uncertainty and increasing the utility of the data globally. An interesting and promising idea is the implementation of AI and machine learning in the process of creating models and simulations. However, the use of AI still requires deeper investigation.