We would like to invite paediatric intensive care units (PICU) to join our multi-center trial concerning patient population < 12 y/o and aiming at:
validation of computed tomography angiography (CTA)/computed tomography perfusion (CTP) tests for brain death/death by neurological criteria (BD/DNC) diagnosis procedures,
validation of duplex Doppler insonation of extracranial segments of the internal cerebral arteries and the vertebral arteries for choosing an optimal time for CTA/CTP testing.
The study has been already accepted by the Bioethical Commission of the Pomeranian Medical University in Szczecin. Informed consent will be obtained from legal representatives of the patients to perform additional brain blood flow tests.
The most seriously ill patients are treated in ICUs. ICU mortality varies in a range of 10–25% and BD/DNC is the reason for up to 15% of ICU deaths. BD/DNC patient is legally considered to be a dead person and in such case any kind of therapy being futile should be terminated. Supportive therapy of BD/DNC patients may be justified only if there is a chance for procurement of transplantable organs. We assume recruitment of 30–40 patients below 12 y/o with BD/DNC diagnosis per year.
Death and BD/DNC diagnosis is a complex issue including medical, religious, and cultural aspects. It was the reason for the development and periodic reviews of BD/DNC criteria in most of the countries. National guidelines for BD/DNC diagnosis are not uniform [1, 2]. There is a worldwide tendency to perform BD/DNC recognition based on meticulous clinical investigation, but in rare cases, in the presence of confounders such as intoxication, infratentorial brain lesions, massive facial injury, or inability to perform apnoea test, usage of ancillary tests is recommended worldwide [2].
The emotions are obviously extremely high in case of paediatric death. Polish physicians would feel more comfortable and safer if they could demonstrate the results of ancillary tests confirming BD/DNC diagnosis. Brain blood flow tests are the most reliable.
There are four brain blood flow tests recommend-ed for BD/DNC diagnosis:
catheter digital subtraction angiography (DSA),
cerebral perfusion scintigraphy,
transcranial Doppler ultrasonography (TCD),
CTA and recently CTP.
Usage of DSA, still considered to be a gold standard and reference method, is gradually decreasing in most countries. It is invasive, requires procedural skills, time and availability of an angiography suite. Besides, it may be additionally technically difficult, especially in a group of small patients. TCD, a completely non-invasive technique, was validated to dia-gnose cerebral circulatory arrest (CCA) in adults and children > 2 years of age. It was implemented in Polish BD criteria in 2007 and maintained in the amendment in 2020 [3]. The sensitivity of TCD exceeds 90% and the specificity is 100% but it is not recommended in children with open fontanelle because of lack of validation studies. Despite its obvious advantages, TCD is not frequently used in Poland because it has to be performed with unique devices which currently are not widely available. Additionally, it is highly operator-dependent and needs certification of the performing physician. Therefore, this method currently can’t be regularly used in BD/DNC diagnostic procedures. The last of “traditional” cerebral perfusion tests – scintigraphy, is rarely available.
In this difficult situation, it became of crucial importance to introduce an ancillary test that would be less invasive than DSA, easily available, easy to perform and interpret. CTA and CTP seem to be the tests of choice for the future [2]. Modern multi-slice CT scanners are fast enough to visualize vasculature and perfusion of the whole brain with a single intravenous injection of iodinated contrast medium and finally confirm CCA. Both CTA and CTP performed with calibrated intravenous contrast injection and precise scanning protocols are operator-independent. Interpretation of CTA based on a 4-points score is relatively easy and final results may be obtained almost immediately. Interpretation of CTP is more complicated and time-consuming. Besides, it requires a dedicated software and radiologist with experience in CTP analysis. Nevertheless, despite these disadvantages, CTP may be more accurate compared to CTA and decisive in some borderline cases [4].
In a recent comprehensive publication, covering all aspects of BD/DNC, completed with 17 supplements, Greer et al. [2] stated that currently available data are not sufficient to recommend CTA/CTP for the diagnosis of CCA and that these methods should be validated in comparison to “gold standard” cerebral perfusion tests such as scintigraphy or DSA. We share this opinion in the aspect of precise validation, but contrary to it, we claim that we had sufficient scientific evidence to introduce these methods into Polish BD/DNC national criteria and recommend it for the other countries.
The research group from the Pomeranian Medical University in Szczecin was involved in scientific projects concerning BD/DNC diagnosis and legislation since the implementation of the first Polish BD criteria in 1984. Our main fields of interest concerned reliability and safety of various methods of apnoea test [5–7] as well as investigation of new techniques for CCA diagnosis [4, 8, 9].
In 2007 we organized a national multi-center trial N N403 171137 entitled “Evaluation of CT angio-graphy and CT perfusion in BD/DNC diagnosis” in a group of adult brain-dead patients. This was followed by a series of publications.
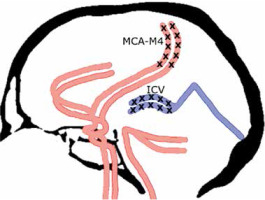
After meticulous analysis of different evaluation scales for CTA in BD/DNC diagnosis procedures [9], we finally accepted the 4-point scale proposed by the French guidelines for the diagnosis of BD/DNC [10]. According to the 4-point scale, CCA may be confirmed if there is a bilateral absence of contrast filling in cortical segments of the middle cerebral arteries (MCA-M4) and internal cerebral veins (ICV) as presented in Figure 1. Unilateral opacification of one or two cortical branches of the MCA does not preclude the diagnosis of CCA as long as the contrast does not fill ICVs.
FIGURE 1
Four-point score criteria of CCA in CTA imaging. Positive result in the 4-point scale (score = 4) confirming CCA is stated when the bilateral MCA–M4 and the bilateral ICV are not opacified
CTP criteria for CCA were not published before and therefore we developed an original instruction based on analysis of cerebral blood flow (CBF) in 1-cm2 circular regions of interest (ROIs) including the midbrain (2 ROIs), the pons (2 ROIs), the medulla oblongata (2 ROIs), the cerebellum (8 ROIs), cortical regions of the frontal (12 ROIs), parietal (12 ROIs), temporal (12 ROIs), and occipital lobes (8 ROIs), as well as the basal ganglia (8 ROIs) drawn bilaterally and placed on each 10-mm axial slice [4] as shown in Figure 2. We recognized CCA in CTP examination if CBF value was below 10 mL 100 g-1 min-1 which is an established value for neuronal necrosis compliant with data published by Astrup et al. [11].
FIGURE 2
Criteria of CCA in CTP imaging. Axial sections of brain with marked positions of ROIs. Colour scale illustrates range of CBF (mL 100 g-1 min-1). CBF < 10 mL 100 g-1 min-1 confirms CCA

We noticed, that in some borderline cases, despite opacification of cortical segments of the MCA in CTA imaging CBF presents values below 10 mL 100 g-1 min-1 and in such cases, it is considered more sensitive and superior to CTA in CCA diagnosis and overall may increase the sensitivity of testing (Figure 3) [4]. We determined CTA sensitivity twice, first CTA validated against DSA reached the sensitivity of 96.3% in a group of 82 BD/DNC patients [9]. In the second publication, we reported CTA sensitivity of 86% and CTP sensitivity of 100% in a group of 50 BD/DNC patients [4]. Validation of CTA/CTP was performed against BD/DNC diagnosis based on clinical examination and supported by TCD in the vast majority of cases, but TCD data were not analysed in this publication [4]. Following this evidence, we concluded that we have enough data for the implementation of CTA/CTP as valid brain blood perfusion tests into guidelines for BD/DNC diagnosis. On the basis of these data, we included CTA/CTP in the Polish national guidelines for BD/DNC diagnosis in patients over 12 years of age [3]. This was the first application of CTP to official BD/DNC diagnosis guidelines.
FIGURE 3
Different CTA (upper row) and CTP (lower row) patterns during CCA diagnosis procedures: A) patient with suspected ischemic stroke with normal CTA and CTP; B) patient with opacification limited to proximal segments of middle cerebral arteries MCA–M1 in CTA (red arrows) and CBF value < 10 mL 100 g-1 min-1 in CTP; both techniques confirm CCA. C) Patient with bilateral opacification of cortical arterial segments (MCA–M4; red arrows) in CTA, not consistent with CCA diagnosis, and CBF value < 10 mL 100 g-1 min-1 in CTP, which confirms CCA diagnosis; D) patient with opacified MCA–M4 segments (red arrows) and opacified internal cerebral vein (blue arrow) in CTA, not consistent with CCA diagnosis, and CBF value < 10 mL 100 g-1 min-1, which confirms CCA diagnosis

There are no reports about the usefulness of CTA and CTP in paediatric patients. Our current experience is limited to a few cases so far, including neonates. Therefore, validation of these tests for the diagnosis of CCA in this group will be a valuable contribution to science and very important for practical aspects.
CCA is not a rapid phenomenon, as reduction of perfusion appears gradually and not simultaneously in all brain regions. Clinical symptoms of BD/DNC, brain stem areflexia, appear due to brain stem hypoperfusion. This does not mean that blood flow is absent in all other parts of the brain, therefore, according to Polish guidelines, CTA/CTP should be performed at least 6 hours after the onset of brain stem areflexia [3]. To avoid unnecessary preterm transportation of the patient to the radiology department and useless examination, duplex Doppler visualization of extracranial segments of the internal carotid and the vertebral arteries may be used. This method may provide valuable information for choosing the right time for CTA/CTP. According to our knowledge, duplex Doppler examination is increasingly available, and therefore it may be possibly used as an additional tool in the BD diagnostic process.
We hypothesize that the patterns of CCA examined with CTA/CTP in children under 12 years of age would be in principle similar as in a group of older patients with possible differences caused by smaller size, pliable skull with open fontanelle, lower blood pressure, and faster heart rate as it was often mentioned in comments concerning paediatric population [2, 12]. Our hypothesis, if confirmed, would allow recommendation for implementation of these less invasive and less operator-dependent ancillary tests for BD/DNC diagnostic procedures.
Aim of the study was to verify our hypothesis, we designed a research project aiming to validate CTA/CTP in a group of BD/DNC children < 12 y/o. The second aim of our study is to assess, whether the duplex Doppler method may be useful for choosing a proper time for CTA/CTP in this population.
METHODS
Study participants
Study participants will be recruited from consecutive (presumably 120–150) BD/DNC patients aged up to 12 years old hospitalized in paediatric ICUs in Poland and possibly in foreign hospitals. BD diagnosis will be conducted according to revised Polish BD criteria which were published last year [3] and in foreign hospitals according to local guidelines.
During the procedure of BD/DNC diagnosis, the family will be asked for informed consent to perform two additional brain blood perfusion tests: duplex Doppler and CTA/CTP examinations. Following BD/DNC confirmation the patients will be declared dead. Patients included in the study will be secondarily divided into three groups:
neonates and infants with open fontanelle – appro-ximately up to 2 years of age,
children from 2 up to 6 years of age,
children from 6 to 12 years of age.
In cases of lack of parental consent for additional brain blood perfusion tests, or the presence of unstable blood pressure refractory to catechola-mine infusion, the patient will be excluded from the study.
Duplex Doppler data acquisition
Duplex Doppler examination of both vertebral arteries and both internal carotid arteries in the extra-cranial segments will be performed to determine a proper time for CT tests. The following types of blood flow will be considered as suggesting CCA: reverberating flow (oscillating, biphasic) with short forward systolic complex and diastolic flow in opposite direction during all diastolic phase, or early systolic peaks, or no demonstrable flow. The recordings of each duplex Doppler examination will be analys-ed by Dr W.J. – the Lecturer of the Neurosonological Section of Polish Neurological Society.
Computed tomography angiography/computed tomography perfusion data acquisition
Data will be acquired using a multi-slice CT scanner after administration of iodinated contrast material at the dose of 1 ml per kilogram of body weight at maximal flow rate achievable for a venous access line (1–4 mL s-1) via an automatic injector followed by the same volume and flow rate of saline solution. Series of scans will be performed for 60 s and will be started with a delay of 4 s after contrast injection. The total coverage in the z-axis will be adjusted for the whole brain volume. The patient’s chin will be tilted toward the chest to shorten the z-axis distance between the skull base and the vertex. This tucked position enables the investigator to visualize the whole brain from the level of the foramen magnum to the vertex.
In participating centers not equipped with CT scanners capable of performing whole-brain CTP, only CTA will be performed according to the revised Polish BD/DNC criteria:
initially, a spiral scan without contrast enhancement covering the range from the level of the C1–C2 vertebra to the vertex will be conducted. The thinnest achievable slices will be used, ≤ 1.5 mm;
thereafter two spiral scans will be completed following administration of a contrast agent with an iodine concentration of at least 350 mg mL-1 at a dose of 2 mL per kilogram of body weight using an automatic injector with a flow rate depending on the diameter and type of intravenous access followed by the same volume and flow rate of saline solution;
immediately after administration of the contrast medium:
the study will cover the range from the C5–C6 level (including division of the common carotid artery) to the vertex. The thinnest achievable slices will be used, ≤ 1.5 mm.
Postprocessing
Perfusion parameters will be calculated in 5 mm axial slices using the commercial perfusion software packages. Processing will be performed semiautomatically with the default settings used in routine clinical practice. To determine perfusion values circular regions of interest (ROIs) will be placed in the brain stem, bilaterally in the frontal, parietal, temporal, and occipital lobes, as well as in the basal ganglia and the cerebellar hemispheres (Figure 2).
The CTA images will be automatically reconstructed from the CT perfusion source images as timing-invariant CTA (TI-CTA). The TI-CTA provides angiography by overlapping all timeframes and displaying maximal enhancement over time. Because of a choice of the temporal maximum, this technique is timing invariant, which means that the maximal enhancement of a vessel is displayed independent of its contrast arrival time. Therefore, TI-CTA is not sensitive to delayed contrast material arrival in cerebral vessels and thus should display any vessel present. This technique was previously described and shown to be reliable by Smit et al. [13].
Image analysis
A neuroradiologist with experience in interpreting cerebral CTA/CTP and blinded to the results of clinical tests will evaluate all images.
For each CTP ROI, the following parameters will be recorded: CBF, CBV, and contrast-to-noise ratio (CNR). The CNR will be calculated using the formula CNR = (peak HU mean − baseline HU mean)/baseline HU SD, where HU represents Hounsfield units and SD represents standard deviation.
CTP findings will be interpreted as consistent with BD/DNC diagnosis (i.e., positive) when CBF and CBV values in all ROIs will be below the well-established thresholds for neuronal necrosis, that is, 10 mL 100 g-1 min-1 and 1.0 mL 100 g-1, respectively [11]. Sawicki et al. proposed these criteria in recent publications concerning CCA diagnosis [9].
The CTA images will be analysed first for the appearance and disappearance of contrast media in the superficial temporal artery branches to confirm that the contrast was injected properly and to eliminate the potential influence of hemodynamic perturbations. The presence of contrast in the different segments of the intracranial arteries will be analysed using CTA according to the 4-point scale based on the lack of opacification of the cortical segments of the MCA and the two ICVs. A score of 1 was given for each of the non-opacified vessel segments.
The CTA findings will be interpreted as consistent with CCA (i.e., positive) if the exam will reveal bilateral non-filling of cortical segments of the MCA and bilateral non-filling of the ICV, or, alternatively, unilateral opacification of one or two cortical branches of the MCA without opacification of ICVs (score = 4).
TI-CTA derived from CTP data will be interpreted according to the same criteria as conventional CTA.
Data collection
The following demographic and clinical data will be collected: age, gender, and cause of brain injury categorized as vascular, traumatic, or anoxic-ischemic,haemodynamic data during clinical and instrumental examinations, anonymized epicrisis and anony-mized BD diagnosis protocol. These data will be stored in encrypted space on servers belonging to the Pomeranian Medical University in Szczecin.
The blood flow data panel will include types of blood flow pattern in extracranial arteries registered by duplex Doppler examination, cerebral blood flow data including cerebral perfusion values and bilateral opacification of the cortical branches of the MCA as well as opacification of ICV.
In case of positive verification of diagnostic accuracy of CTA and CTP for confirmation of CCA in paediatric patients, these methods will be recommended for implementation into the panel of ancillary tests used in BD/DNC diagnostic procedures.




