Introduction
Prostate cancer is the second most commonly diagnosed malignancy and the fifth leading cause of death from cancer in men worldwide [1]. Despite prostate-specific antigen (PSA)-based screening and early detection guidelines, approximately 15–26% of prostate cancer patients present with high-risk (HR) features indicative of a more advanced and potentially lethal course [2]. Patients with more advanced or poorly differentiated tumors could also potentially benefit from surgery [3]. Interest in radical prostatectomy (RP) as a treatment option for patients with HR disease has increased over the past decade. This trend stems from growing evidence that surgery, either alone or in combination with adjuvant treatments, is associated with favorable cancer control outcomes [4, 5]. Intuitively, surgery may be more beneficial when complete removal of the disease is possible, in patients with tumors confined to the RP specimen. This hypothesis is supported by more favorable survival rates in HR prostate cancer patients harboring organ-confined disease at the RP [6]. Guidelines from the European Association of Urology (EAU) now support a role of RP in the treatment of select HR patients, which may include a multimodal approach [7]. The goal of surgery in a HR patient is to achieve a good oncological outcome. Surgical treatment includes extended lymph node dissection (ePLND), clean apical dissection, neurovascular bundle resection on the tumor-bearing side, complete resection of the seminal vesicles, or resection of the bladder neck [8]. A radical procedure consists of two operations performed at the same time, involving prostate removal and ePLND. Surgery can be performed by either a transperitoneal or an extraperitoneal approach. However, transperitoneal access seems to be more advantageous because higher rates of complications, such as symptomatic lymphoceles, appear to be attributed to the extraperitoneal approach [8].
Aim
The aim of the study is to present our new combined technique of RP using two different approaches: a pre-peritoneal approach for laparoscopic radical prostatectomy (LRP) and a transperitoneal approach for ePLND.
Material and methods
This prospective study included 30 patients aged 53 to 75 years (mean age: 64 years) diagnosed with prostate cancer who qualified for LRP with ePLND by combined technique. We developed a new technique for the extraperitoneal approach to remove the prostate first and a transperitoneal approach for ePLND after prostatectomy. All patients were qualified for invasive treatment according to EAU prostate cancer guidelines: intermediate-risk patients with a pre-operative risk nomogram indicating > 5% likelihood of lymph node metastasis and all high-risk patients. Every participant was informed about this technique, received an explanation of the details, and provided signed informed consent. All of the procedures were performed by two experienced urologists. We evaluated all histopathological parameters before and after the operation, procedure details, and operator’s comments about the combined approach.
Pre-operative prostate volume measured by magnetic resonance imaging ranged from 20 to 122 ml (mean: 47.1 ml) and PSA values from 4.3 to 54.29 ng/ml (mean: 17.04 ng/ml). The clinical stage was cT2 in 14 patients and cT3 in 16 patients. The degree of malignancy according to the Gleason scale (GS) was dominated by GS ≥ 8 (23 patients, 77%; Table I). According to the 2014 classification of the International Society of Urological Pathology (ISUP), ISUP grade ≥ 4 was predominant (19 patients, 63%). There were no patients with suspicion of metastasis in MRI or bone scan imaging. Multiparametric magnetic resonance imaging (mpMRI) was used to measure changes on the Prostate Imaging and Reporting and Data System (PIRADSv2) scale; 28 (93%) patients were PIRADS 4 or 5. In the mpMRI study, 13 patients were suspected of extracapsular extension (ECE) and 10 lymph node invasion (LNI). The probability of lymph node (LN) metastasis according to the Briganti nomogram > 5% concerned 25 (83%) patients. ePLND was performed in these patients. For the remaining 5 patients, the decision to execute ePLND was due to suspicion on multi-parametric magnetic resonance imaging (mpMRI) of LNI and/or ECE (Table I). Each time, the type of procedure was discussed with the patient, who made the final decision and gave written consent for the procedure to be carried out.
Table I
Preoperative characteristics of the patients
Operative technique
Thirty patients were planned for RP with expanded pelvic lymphadenectomy performed by a standard LRP technique, a combined technique to remove LNs. The operation was performed using both extraperitoneal and transperitoneal approaches simultaneously.
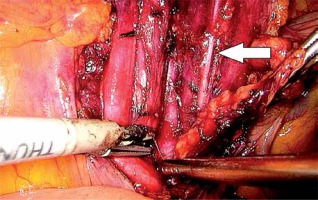
In the first stage, RP was performed using pre-peritoneal access, preserving all the rules of the procedure for HR prostate cancer. Five trocars were used in typical places. After the RP, ePLND was performed according to our own technique. In the first stage, transposition of the trocars was performed. First, a trocar placed under the peritoneum was inserted under the navel for optics. CO2 was administered through this trocar, insufflation performed, and the remaining three trocars transposed into the peritoneal cavity under vision control without changing their location. The exception was the extreme left trocar, which was inserted through the new opening above the drain previously left in the space before retardation. In the pre-peritoneal space, the tube was left in place of the 5 mm trocar, the one furthest on the left. The drain in the pre-peritoneal space remained open during the entire second stage of pelvic LN removal (Photo 1). Removal of the pelvic LNs was then initiated. First, we identified the iliac vessels and ureter crossing the common iliac artery (Photo 2). All treatments proceeded according to the same scheme, starting from the right. The presacral LNs were always removed first. The procedure began by dissecting the peritoneum medially from the right ureter (Photo 2). The presacral nodes were then removed from the promontory to reveal the median sacral artery (Photo 3). The median sacral artery arises just above the aortic bifurcation into the common iliac arteries. Next, we moved medially from the vein and common iliac artery towards the triangle of Marcille (the lumbosacral triangle) (Photo 4). The tissue was removed from the common iliac artery and the ureter dissected. The proximal extent of dissection was controlled with 5 mm hem-o-loc clips and divided. The external iliac artery was skeletonized from the origin of the artery and nerve genitofemoralis (Photo 5). The limits of dissection were the common iliac proximally to the inguinal ligament (the node of Cloquet) distally. In the next stage, we proceeded to prepare the obturator hole. The obturator nerve, which is on the floor of this dissection, was carefully preserved. We did not cut the vas deferens. The vasa deferentia were retracted, providing excellent visualization of the LN packets. At the end of the internal iliac, the group of nodes was removed. Lymphadenopathic tissue surrounding the internal iliacs and branches was also removed. Nodal tissue around the internal iliac often coalesced with the obturator packet anteriorly and posteriorly to the lateral sacral nodes. The limit of the dissection was the lateral sacral arteries arising from the posterior division of the internal iliac vein (Photo 6). The tissues on the left side were removed in the same way and the procedure terminated by leaving the second tube in the vesiculo-rectal recess.
Results
All 30 patients underwent LRP and ePLND using a combined technique. The results of histopathological examination were as follows: adenocarcinoma of the prostate, stage: T2a – 2, pT2c – 8, pT3a – 6, pT3b – 14; for grades of malignancy according to the Gleason scale and ISUP (Table II). A positive margin was found in 5 (16%) patients, including 3 patients out of 13 with extracapsular extension based on mpNMR. The total duration of surgery was 155 to 290 min (mean: 215 min); the pelvic lymphadenectomy lasted from 35 to 85 min (mean: 56 min; right side: 20–45 min, mean 30 min; left side: 15–40 min, 26 min). The mean blood loss was 180 ml (200–400 ml). The movement of trocars into the peritoneal cavity was a very simple maneuver, taking up to 1 min without any complications. We recognized positive nodes in 9 (30%) patients. The number of removed LNs ranged from 13 to 28 (mean: 16.8). The most common location of metastatic lesions was the internal iliac junction (11 nodes), followed by obturator (8 nodes), presacral (5 nodes), and external iliac (4 nodes). The drain from the pre-peritoneal space was removed in the first 24 h. The drain from the peritoneum was removed when lymph leakage fell below 300 ml and the same day patients were discharged home. Six weeks after surgery, PSA was undetectable in 23 (77%) patients. In 7 patients, the PSA level ranged from 0.09 to 10.0 ng/ml (3 patients from 0.1 to 0.5 ng/ml, and 4 > 0.5 ng/ml). In 11 (36%) patients, radiotherapy and/or hormone therapy was recommended.
Table II
Postoperative characteristics of the patients
Complications
We recorded complications in three patients. One patient suffered bleeding into the peritoneal cavity from the kidney vessels and underwent surgery and blood transfusion. In another patient, a leaky anastomosis was identified. The third patient suffered a subcutaneous pneumothorax, which was treated conservatively.
Discussion
Though the role and potential benefits of ePLND in prostate cancer are still open to debate, it provides accurate staging and remains the accepted and most effective method for detecting LN metastases and is the gold standard for assessing nodal status in prostate cancer [7–10]. The decision to extend the operation to remove nodes must be dictated by reasonable grounds. In our study, we aimed to present a combined technique using both an extraperitoneal and transperitoneal approach for ePLND, and confirm that such access can be safe for the patient and ergonomic for the surgeon.
Several publications have suggested that the extent and quality of a pelvic LND are not dependent on a specific surgical technique. Rather, nodal yield is far more dependent on surgeon’s intent than the technical approach (open, laparoscopic, or robotic) [11]. At our center, all HR prostatectomies are performed by experienced operators. When deciding on a combination of LRP + ePLND, we always choose transperitoneal access, avoiding pelvic lymphocele, the most frequent complication after ePLND [12].
In 30 patients, we decided on a combined technique, which simultaneously used both access routes: a retroperitoneal access to remove the prostate and transperitoneal access for LN removal. In all 30 patients, we first carried out prostatectomy using pre-peritoneal access in the conventional manner; we then moved the trocars into the peritoneum for lymphadenectomy. The movement of trocars into the peritoneal cavity was a very simple maneuver, taking up to 1 minute. During prostate removal, we paid special attention to the 13 patients in whom mpMRI indicated infiltration of the capsule. In the final histopathological examination, the presence of positive margins was confirmed in only 5 (16%) patients versus 35% (range: 12–53%) described in the literature [13]. Preservation of oncological purity by avoiding positive margins is a key condition for successful surgery. This is particularly difficult in HR patients with locally advanced cancer.
Combined technique also required modifying the position of the drains. We left a drain in the retroperitoneal space (in place of the left lower trocar) after completing the first stage of prostatectomy. The drain remained open throughout the course of the second stage, lymphadenectomy. There were exceptions to this practice in the case of 2 patients due to communication between spaces; in these patients, the drain remained closed. On the right side of these patients, we introduced an additional trocar with a 5 mm traction tool to lift the bladder. The second stage began by repositioning the first trocar for optics to the peritoneal cavity. This maneuver was easy because the peritoneum was always exposed during the creation of the retroperitoneal space. The introduction of insufflation into the cavity caused the retroperitoneal space to fall; the subsequent stage involving lymphadenectomy did not differ from the classical procedure. The time required for ePLND ranged from 35 to 85 min (mean: 56 min). In a previous study, Katz et al. estimated that the operative time for PLND was 47 min in cases of standard PLND and 66–86 min (mean: 72 min) in cases of ePLND [14] Similar times were reported by other authors. Yuh et al. reported a time of 30–45 min [15], whereas Davis et al. quoted a time of 36–50 min [16]. However, in the current literature, only a few authors have described application of the extended PLND; papers have been confined mostly to limited or standard lymph node dissection (LND) [13]. During the removal of nodes, we always used the same scheme, beginning with dissection of the presacral lymphatics. Separate packets were sent from the perisacral, bilateral common iliacs, external iliacs, internal iliacs, and obturator. Heidenreich et al. suggested that it is unnecessary to dissect the presacral and common iliac lymphatics, as only 3.1% of patients have LN metastases in that region [17]. However, in the present study, we identified metastases in up to five presacral nodes in 2 patients.
After removal of the presacral nodes, we moved along the common iliac artery, separating the ureter, and then along the external iliac artery to the node of Cloquet, which forms the upper boundaries of the preparation. The external border was the genitofemoral nerve. Initially, we were concerned that after the first retroperitoneal stage of prostate removal it would be difficult to carry out transperitoneal access to the presacral nodes and reach the triangle of Marcille [18]. However, we did not experience any difference between the classic and mixed operations except in two patients with pre-peritoneal and transperitoneal communication. We then used the traction tool introduced by the additional trocar to raise the bladder. Avoiding disturbance of the peritoneum during the first stage appears to be a key element of this procedure. This is not difficult, and we managed to carry it out successfully in 28 patients. The border for tissue removal along the internal iliac artery was the lateral sacral artery.
In a previous study, Jung et al. found that 25% of metastatic LNs are located in the internal iliac and common iliac regions [19]. The internal iliac nodes may be a primary landing zone, as 3 of 4 patients had exclusively internal iliac node metastasis [20]. This was also confirmed by our studies in which the most common location of metastases was the area of the internal iliac veins (11 positive and 8 subsequently positive obturator nodes). However, Mattei et al. reported that most LNs (38%) were located alongside the external iliac and obturator packets [21]. These authors also found that 25% of primary lymphatic drainage occurs via the hypogastric nodes and 16% via the common iliacs vessels
All treatments involving our current patients were standard, and blood loss ranged from 200 to 400 ml. There was no need for intraoperative blood transfusion in any of our patients. Similar results have been published previously: 200 ml (150–800 ml) [22] and 300 ml (150–400 ml) [22]. However, in the present literature, only a few authors have actually published the use of ePLND rather than standard LND [13, 23]. According to the Clavien-Dindo classification, we observed grade 3 complications in three patients. In one case, we decided to re-operate.
One stage of RP for HR prostate cancer is entire prostate gland removal, which is done under operator-friendly conditions. The procedure is carried out in the retroperitoneal space with high CO2 pressure, which helps in hemostasis, with no pushing of the bowels, offering a good working space. In addition, performing anastomosis in the retroperitoneal space results in excellent visualization. The second stage of RP, ePLND, is much more feasible when performed via the transperitoneal approach. In the traditional technique, the very last part of RP (anastomosis) is the most difficult. During our combined technique, this stage is performed at the end of the first part of the operation (in the middle of the whole of procedure), when the surgeon is not as tired as at the end of long-lasting HR prostate cancer RP. This gives the opportunity to focus on meticulous anastomosis, which offers good functional results.
The main limitation of the present study is the small number of cases and short observation period. However, our encouraging results confirm the potential of combined techniques in the future.
Conclusions
The combined technique is both feasible and safe. Good oncological purity, as measured by the number of negative margins and functional results, confirms the validity of the application of our combined technique. Performing the most difficult maneuver in HR prostate cancer (removal of the prostate in the first stage) appears to be more convenient for the operator. The timing of ePLND stage in the combined technique and the number of removed LNs do not differ from the standard lenticular access. Further comparative testing is necessary to demonstrate the clinical advantages of this new procedure.