A 77-year-old male patient presented with heart failure symptoms (NYHA class III), which developed after probable infective endocarditis (IE) a few months earlier. Physical examination revealed new heart murmur and peripheral oedema. His other medical history includes hypertension, laryngeal tumor, and spine surgery.
Nine years prior to this presentation, he was qualified for surgical aortic valve replacement (SAVR) due to a symptomatic severe aortic stenosis with mild aortic regurgitation. After sternotomy, he was deferred from prosthesis implantation as the porcelain aorta was revealed. Subsequently, he underwent successful transcatheter aortic valve implantation (TAVI) with Medtronic CoreValve 29 mm (Medtronic, Minneapolis, MN, USA) bioprosthesis. Postprocedural transthoracic echocardiography (TTE) showed proper bioprosthesis function with maximal transaortic gradient of 25 mm Hg without a paravalvular leak (PVL). The postoperative course was uneventful and on the 7th day he was discharged home.
Three weeks after the procedure, the patient developed recurrent syncope, dizziness and chest pain. Electrocardiogram showed severe bradycardia with periodic third-degree atrioventricular block. A dual chamber pacemaker (BiotronicEcuro DR, Biotronik, Berlin, Germany) was implanted.
During next 9 years the patient remained in a good clinical condition, though he underwent successful treatment of laryngeal tumor. The repeated echocardiographic examination confirmed proper bioprosthesis function. Six months before current hospitalization he presented with infection which was interpreted as pneumonia and treated successfully with antibiotics. Nevertheless, thereafter symptoms of heart failure occurred and gradually exacerbated.
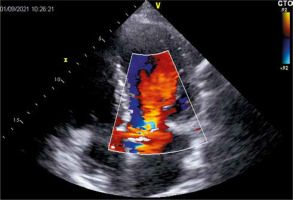
During the hospitalization due to heart failure worsening, TTE revealed hemodynamically severe intraprosthetic aortic regurgitation which had not been observed before (confirmed in transesophageal echocardiography (TEE) (Figure 1)) with transaortic maximal and mean gradients of 21 and 10 mm Hg, respectively, preserved left ventricular ejection fraction, and increased pulmonary artery systolic pressure. Laboratory tests did not present significant deviations in inflammatory markers and blood cultures were negative.
After careful assessment by the heart team the patient was qualified for valve-in-valve TAVI (ViV-TAVI) due to a high perioperative risk and porcelain aorta. After careful assessment of computed tomography, he underwent successful uncomplicated implantation of Edwards Sapien (Edwards Lifesciences Corp., Irvine, CA, USA) prosthesis, which was chosen based on its optimal profile not limiting access to the coronary ostia. During follow-up he remains asymptomatic with good prosthesis function on control echocardiography.
IE post-TAVI is not a common but serious complication with high in-hospital mortality, which is estimated at about 11–64% and 1-year mortality of 22–75% [1]. The overall incidence of IE post-TAVI is about 1% to 6% [2].
The rate of IE post-TAVI 1 year after surgery ranges from 0.5% to 3.1% and will increase as the procedure gains popularity and the operated patients are older and with a high operational risk [2]. According to several reports, the most common etiologic factors causing IE post-TAVI include Enterococci, Staphylococcus aureus, and coagulase-negative Staphylococcus species, accounting for approximately 26%, 16%, and 15% of cases, respectively [3, 4].
Diagnosing IE post-TAVI is very challenging because of the diversity of the clinical presentation and nonspecific symptoms, especially in elderly patients with comorbidities.
The most common symptoms reported by patients are fever (80%) and heart failure (22%) [2], and these were also presented by our patient during his infection identified as acute bronchitis and treated with antibiotics. Less frequent symptoms include embolic events, general malaise, weakness or weight loss. Our patient described progressive physical decline after an episode of bronchitis.
The main sources of IE post-TAVI are respiratory infections, dental interventions, skin infections, and urological or gastrointestinal interventions. However, in half of the patients, the cause remains unidentified [3]. In our patient, it is difficult to retrospectively establish if the bronchitis was a source of bacteriemia or masked the acute IE.
Echocardiography and blood cultures play a major role in diagnosing IE and should be performed as soon as the disease is suspected. However, the examination may be difficult in subtle forms or without evident changes, and negative echocardiographic result may occur in even 15% and lead to delays in the initiation of appropriate therapy [3]. The difficulties may be mainly explained by the shadowing of the stented frame abutting the native valve leaflets [5] and the presence of calcifications of the residual native valve tissue. Nevertheless, given the above case presentation and mentioned facts of types of echocardiographic findings, we believe that echocardiography should be certainly performed in patients after TAVI who present symptoms of any severe infection, such as pneumonia, and particularly with subsequent symptoms of heart failure, to exclude or confirm IE. having in mind that IE is a potentially life-threatening complication, and may lead to potential prosthetic valve degeneration, echocardiography, even if may seem irrelevant in some patients, may occur crucial. In cases with doubtful TTE results, however, with a high clinical suspicion, TEE should be considered, and blood samples’ cultures are beneficial.
Treatment of IE post-TAVI is based on antibiotic therapy and, in selected patients, surgery, however the exact recommendation how to manage this condition remains unclear and still challenging. Importantly, surgery is difficult after TAVI, but not impossible, and should be performed by highly experienced cardiac surgeons. A careful assessment of each patient by the heart team is crucial. In very selected cases, it is possible to perform a ViV-TAVI procedure after antibiotic therapy and confirmation of negative blood cultures. There are currently no randomized trials proving the superiority of one of the surgical methods (Redo-SAVR vs. ViV-TAVI) in the treatment of degenerated aortic valves, therefore the idea of ViV-TAVI as a less invasive method is interesting.
ViV-TAVI is a relatively safe procedure for patients with an intermediate and high operational risk with degenerated surgical bioprosthesis compared to surgical technique [6], though still requires high operative experience. According to the PARTNER 2 trial, advantages of ViV-TAVI include relatively low complication and mortality rates, improved hemodynamics, and optimal functional and quality-of-life outcomes at 1 year [7].
Redo-SAVR was not chosen due to the porcelain aorta in our patient and high perioperative risk. Redo-SAVR compared to ViV-TAVI seems to deal with several difficulties due to deep anesthesia, re-sternotomy, cardiopulmonary bypass, aortic cross-clamp, blood transfusion requirement, longer intubation time and longer intensive care unit and hospital stay.
A recent meta-analysis which compared Redo-SAVR vs. ViV-TAVI showed that the incidence of all-cause mortality after ViV-TAVI is significantly lower within 30 days. This advantage was not relevant between 30 days and 1 year, and 1 year after intervention Redo-SAVR shows a better outcome [8]. However, these studies have some limitations as patients who underwent ViV-TAVI have a higher surgical risk compared to Redo-SAVR, and many of them were disqualified from conventional surgery.
In conclusion, we underline the necessity of echocardiography and clinical mindfulness in patients with previous TAVI presenting with symptoms of acute infection, and particularly those who report progressing clinical deterioration. ViV-TAVI is a considerable option for patients with healed IE, when performed by a highly experienced TAVI team.