Introduction
In June 2023, a multi-society Delphi consensus introduced the term metabolic dysfunction-associated steatotic liver disease (MASLD) instead of non-alcoholic fatty liver disease (NAFLD) [1].
Metabolic dysfunction-associated steatotic liver disease is one of the most frequent chronic liver diseases in the world [2]. MASLD is a broad category of hepatic pathological conditions ranging from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH) with or without fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [3]. MASLD has been defined as the liver manifestation of metabolic syndrome. It is closely linked to obesity, insulin resistance (IR), type 2 diabetes mellitus (DM), hypertension, and hypertriglyceridemia [4]. The complex interaction between some or all of these factors leads to the accumulation of lipotoxic lipid products, which activate liver resident macrophages or Kupffer cells (KCs) [5].
Hepatic steatosis results from an imbalance between increased free fatty acid (FFA) acquisition through hepatic FFA synthesis and uptake and decreased FFA elimination via β-oxidation and very low-density lipoprotein exportation [6]. Lipotoxicity occurs when the liver is stressed by increased fatty acids and other lipotoxic lipid products, causing activation of KCs that release inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-1β and chemokine such as the chemokine (C-C motif) ligand 2 (CCL2) leading to recruitment of bone marrow-derived monocytes, which can differentiate into monocyte-derived KCs and contribute to disease progression. Lipotoxicity leads to endoplasmic reticulum stress, mitochondrial injury, and oxidative stress, thereby leading to lipoapoptosis, the main feature of MASH [5, 7, 8]. Transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), TNF-α, and IL-1β produced by hepatic macrophages activate hepatic stellate cells to become collagen-producing myofibroblasts that secrete extracellular matrix resulting in liver fibrosis [9]. Furthermore, IL-6 and TNF-α contribute to HCC occurrence by activating different oncogenic pathways such as c-Jun terminal kinase, nuclear factor-kappa B signaling, and signal transducer and activator of transcription [10, 11].
Metabolic dysfunction-associated steatotic liver disease is associated with systemic inflammatory conditions caused by increased inflammatory cytokines produced by proinflammatory macrophages that can promote different extrahepatic diseases. These cytokines cause impaired insulin signaling, causing IR and increasing risk for DM development; moreover, they promote liver production of C-reactive protein, plasminogen activator inhibitor-1, and fibrinogen, causing endothelial dysfunction which together with atherogenic dyslipidemia causes progressive cardiovascular disease [4, 12]. Endothelial dysfunction and oxidative stress can induce upregulation of the renin-angiotensin system; also, IR can induce fetuin-A secretion, causing acute kidney injury, and may progress to chronic kidney disease in MASLD patients [13]. Increased risk of extrahepatic malignancies in patients with MASLD may be due to IR that increases the expression of insulin-like growth factor (IGF)-1, which has anti-apoptotic and mitogenic effects [14].
Macrophage activation in MASLD is reflected by increased expression of CD163, which is a hemoglobin-haptoglobin scavenger receptor that is expressed by the macrophages and monocytes; following macrophage activation, shedding of CD163 from the cell surface occurs and is detected in the blood as sCD163 [15]. sCD163 plasma levels are reported in disorders involving macrophage activity, such as acute and chronic inflammation, and are linked to hepatic steatosis, inflammation, and fibrosis [16].
Material and methods
In a case-control study, 30 patients diagnosed with MASLD were referred to the Hepatobiliary Unit. MASLD diagnosis was based on clinical, laboratory, radiological, and histopathological findings consistent with MASLD. Also, 30 age- and sex-matched healthy subjects were included as a control group. Ethical approval was obtained from the institutional ethics committee, and informed consent was obtained from all patients and controls participating in the study. MASLD patients included in the study were ≥ 21 years old with MASLD criteria by ultrasonography. Exclusion criteria were seropositivity for hepatitis C virus (HCV) or hepatitis B virus infection, known causes of other chronic liver diseases, history of alcohol consumption, and any malignancies other than HCC. All patients were subjected to clinical examination and anthropometric measurements (weight, height, and body mass index (BMI) were calculated as body weight in kg divided by height in meters squared and waist circumference), complete blood count (CBC) and liver function tests, including serum alanine aminotransferases (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total serum bilirubin, serum albumin and prothrombin activity. Viral markers including HCV antibodies, hepatitis B surface antigen, and hepatitis B core antibodies were assessed using enzyme-linked immunosorbent assay (ELISA). Lipid profile included serum levels of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG). Fasting blood glucose (FBG), fasting serum insulin (FSI), and homeostatic model assessment for insulin resistance (HOMA-IR) were assessed [17]. Severity of liver disease was assessed by a) fibrosis-4 index (FIB-4) = age (years) × AST (U/l)/(platelets (109/l) × (ALT (U/l))1/2) [18]; b) AST to platelet ratio index (APRI) score (AST/upper limit of normal)/platelet count (109/l) × 100 [19]; c) NAFLD fibrosis score (NFS) = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (×109/l) − 0.66 × albumin (g/dl) [20]; d) BARD score comprised three variables: BMI ≥ 28 = 1 point; AST/ALT ratio ≥ 0.8 = 2 points; and diabetes = 1 point. The possible score ranges from 0 to 4 points [21]. Abdominal ultrasonography was performed.
Measurement of serum soluble CD163 levels
Serum levels of sCD163 were determined quantitatively in patients with MASLD and healthy subjects using a commercially available standard sandwich ELISA kit according to the manufacturer’s instructions (Fine test, catalog No: EH0075), and results were reported as pg/ml [22].
Histopathological examination
Core liver biopsies obtained from patients with MASLD were fixed in 10% formalin solution, embedded in paraffin, cut into 5-µm thick sections, and subsequently stained with hematoxylin-eosin and Mason trichrome stains for the diagnosis of MASLD, and morphologic evaluation for the presence of steatosis, inflammation, and fibrosis using the Brunt system. Activity grade was assessed according to the non-alcoholic fatty liver disease activity score (NAS) using the sum of 3 components (total: 0-8 points):
Steatosis: 0 – < 5%, 1 – 5-33%, 2 – > 33-66%, and 3 – > 66%;
Lobular inflammation: 0 – no foci, 1 – < 2 foci/200× field, 2 – 2-4 foci/200× field, and 3 – > 4 foci/200× field;
Hepatocyte ballooning: 0 – none, 1 – few, and 2 – many.
Fibrosis stage was assessed as follows: stage 0 – none, stage 1 – perivenular (zone 3) or portal fibrosis, stage 2 – perivenular and portal fibrosis, stage 3 – bridging fibrosis; and stage 4 – cirrhosis [23].
Immunohistochemistry for CD163: Immunohistochemical staining of formalin-fixed paraffin-embedded tissue sections was performed by applying the streptavidin-biotin-peroxidase method. The UltraVision detection system (Thermo Fisher Scientific) was used. Tissue sections were deparaffinized and incubated with the following primary antibody at 4°C overnight in a humid chamber: Anti-CD163 Ab-1 (Clone 10D6) – mouse monoclonal antibody (Invitrogen, Thermo Fisher Scientific – Catalog #MA5-11458) at a dilution 1 : 25. Slides were then incubated with biotinylated goat anti-polyvalent (linking reagent), followed by peroxidase-conjugated streptavidin, each for 20 minutes at room temperature. Tissue sections were washed with PBS for 5 minutes after each step. A brown color reaction was developed using 3-3’ diaminobenzidine tetrahydrochloride (DAB) mixture for 10 minutes. The slides were finally dehydrated, counterstained with hematoxylin, and mounted. Negative control sections (where the primary antibody has been omitted) were included in each run.
Evaluation of CD163 immunostaining
The density of cells within the portal tract and liver lobule, which stained positive, was determined by counting the number of CD163-positive cells in 10 random portal tracts and 10 areas of the lobular region at a magnification of 200× under light microscopy, and the mean was calculated [24].
Statistical analysis
IBM SPSS software version 20.0 was used for data analysis. Qualitative data were represented as numbers and percentages. Quantitative data were represented as range (minimum and maximum), mean ± standard deviation and median. The chi-square (χ2) test was used to compare between different categorical variables. Fisher’s exact or Monte Carlo correction for χ2 was used when more than 20% of the cells had an expected count of less than 5. When comparing two groups of normally distributed quantitative variables, the Student t-test was employed. When comparing two groups with abnormally distributed quantitative variables, the Mann-Whitney test was used. Correlation between two non-normally distributed quantitative variables was done using the Spearman coefficient test. Correlation between quantitative variables that were normally distributed was done using the Pearson coefficient. Significance of the obtained results was determined at the 5% level. A receiver operating characteristic (ROC) curve was generated by plotting sensitivity on the Y axis versus 1-specificity on the X axis at different cut-off values. The area under the ROC curve denotes the diagnostic performance of the test. By calculating the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), the accuracy of serum sCD163 level for discriminating MASLD patients from controls was determined.
Results
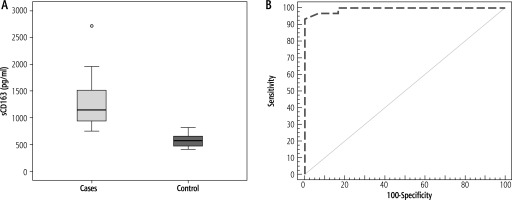
Demographic data and biochemical profiles of participants enrolled in the study are shown in Table 1. The median level of sCD163 was significantly higher in patients with MASLD compared with healthy controls (p < 0.001) (Table 1, Fig. 1A). ROC curve analysis showed that the sensitivity and specificity of sCD163 level in discriminating patients with MASLD from healthy controls were 93.33 and 100.0, respectively, at a cut-off value of 814 pg/ml (AUC = 0.993), and the PPV and NPV were 100.0 and 93.7 respectively (Fig. 1B).
Table 1
Comparison between metabolic dysfunction-associated steatotic liver disease (MASLD) and control groups and between patients with NAS < 5 and NAS ≥ 5 according to demographic and laboratory data
| Demographic and laboratory data | MASLD (n = 30) | Control (n = 30) | p | NAS < 5 (n = 16) | NAS ≥ 5 (n = 14) | p |
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| Male | 3 (10) | 9 (30) | 0.053 | 2 (12.5) | 1 (7.1) | 1.000 |
| Female | 27 (90) | 21 (70) | 14 (87.5) | 13 (92.9) | ||
| Age (years) | ||||||
| Mean ±SD | 44.67 ±12.09 | 39.73 ±10.22 | 0.093 | 47.38 ±12.74 | 41.57 ±10.93 | 0.195 |
| BMI (kg/m2), mean ±SD | 32.79 ±5.14 | 23.73 ±1.5 | < 0.001* | 29.33 ±2.67 | 36.73 ±4.39 | < 0.001* |
| Waist circumference (cm), mean ±SD | 103.7 ±12.1 | 92.5 ±10.3 | 0.001* | 98.06 ±10.55 | 110.21 ±10.58 | 0.004* |
| Hb (g/dl), mean ±SD | 12.66 ±1.31 | 13.2 ±0.92 | 0.071 | 12.94 ±1.47 | 12.34 ±1.06 | 0.209 |
| WBCs (×103/mm3), mean ±SD | 7.67 ±2 | 6.91 ±1.44 | 0.098 | 7.77 ±1.47 | 7.56 ±2.53 | 0.786 |
| Platelet count (×103/mm3), mean ±SD | 281.4 ±73.14 | 296.9 ±59.23 | 0.369 | 268.0 ±68.38 | 296.64 ±77.89 | 0.292 |
| AST (U/l), median (min.-max.) | 22 (14-46) | 20 (16-28) | 0.547 | 22.5 (14-46) | 19.5 (14-42) | 0.208 |
| ALT (U/l), median (min.-max.) | 22 (11-64) | 18 (7-25) | 0.001* | 22.5 (11-58) | 21.5 (13-64) | 0.580 |
| ALP (U/l), median (min.-max.) | 84.5 (52-238) | 76.5 (60-92) | 0.109 | 82.5 (52-238) | 93 (53-165) | 1.000 |
| Albumin (g/dl), mean ±SD | 4.20 ±0.31 | 4.36 ±0.46 | 0.121 | 4.16 ±0.32 | 4.25 ±0.31 | 0.452 |
| Bilirubin (mg/dl), median (min.-max.) | 0.5 (0.2-1.3) | 0.6 (0.2-0.9) | 0.102 | 0.5 (0.2-0.8) | 0.5 (0.2-1.3) | 0.728 |
| Prothrombin activity (%), | ||||||
| median (min.-max.) | 103.8 (80-122) | 105 (90-113) | 0.478 | 104.4 (86.1-14) | 103.2 (80-122) | 0.918 |
| Total cholesterol (mg/dl), mean ±SD | 185.1 ±33.51 | 153.9 ±17.42 | < 0.001* | 185.5 ±33.26 | 184.57 ±35.03 | 0.941 |
| HDL-C (mg/dl), mean ±SD | 43.6 ±5.57 | 46.13 ±5.01 | 0.069 | 43.13 ±6.03 | 44.14 ±5.16 | 0.626 |
| LDL-C (mg/dl), mean ±SD | 111.53 ±24.09 | 83.83 ±5.07 | < 0.001* | 113.31 ±25.69 | 109.5 ±22.91 | 0.673 |
| Triglycerides (mg/dl), | ||||||
| median (min.-max.) | 169 (63-430) | 104 (89-128) | < 0.001* | 125.5 (63-184) | 190 (131-430) | < 0.001* |
| FBG (mg/dl), median (min.-max.) | 94.5 (85-196) | 89.0 (75-99) | < 0.001* | 93.5 (89-115) | 98 (85-196) | 0.208 |
| FSI (µIU/ml), mean ±SD | 16.35 ±7.55 | 5.94 ±1.21 | < 0.001* | 11.53 ±5.66 | 21.85 ±5.39 | < 0.001* |
| HOMA-IR, mean ±SD | 4.27 ±2.26 | 1.29 ±0.26 | < 0.001* | 2.76 ±1.36 | 5.99 ±1.80 | < 0.001* |
| sCD163 (pg/ml), median (min.-max.) | 1144.95 (752-2714) | 568.6 (409.4-814) | < 0.001* | 960 (752-1334) | 1538.7 (987-2714) | < 0.001* |
Normally distributed data are expressed as mean ±SD and were compared using the Student t-test, and abnormally distributed data are expressed as median and were compared using the Mann-Whitney U test; χ2 – Chi-square test was used to compare the two groups as regards sex MASLD – metabolic associated steatotic liver disease, BMI – body mass index, Hb – hemoglobin concentration, WBCS – white blood cell count, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, FBG – fasting blood glucose, FSI – fasting serum insulin, HOMA-IR – homeostasis model of assessment insulin resistance, sCD163 – soluble CD163, NAS – non-alcoholic fatty liver disease activity score
Fig. 1
A) Comparison between the two studied groups according to sCD163, B) ROC curve for sCD163 to discriminate metabolic associated steatotic liver disease (MASLD) cases from controls
The histopathological findings in liver specimens from patients with MASLD are presented in Table 2 and Figure 2. MASLD patients were subdivided according to NAS into patients with NAS < 5 in 16 cases (53.3%) and NAS ≥ 5 in 14 patients (46.7%) (Table 2). Steatosis was predominantly macrovesicular, and zone 3 was the predominantly affected area in most cases (Fig. 2A). Lobular inflammation, hepatocyte ballooning degeneration, and fibrosis stage are presented in Table 2 and Figures 2B, 2C, and 2D, respectively.
Table 2
Histopathological finding of liver biopsies of patients with metabolic dysfunction-associated steatotic liver disease (MASLD) (n = 30)
Fig. 2
A) Mild macrovesicular steatosis, with an azonal distribution (H&E 100×); B) A focus of lobular inflammation (thick arrow). Many of the surrounding hepatocytes show glycogenated nuclei (H&E 400×); C) A contiguous patch of hepatocytes showing prominent ballooning injury (arrows) is seen, sharply contrasted against the surrounding non-ballooned hepatocytes in the field (H&E 200×); D) A case showing bridging fibrosis (arrows) (Masson trichrome stain 200×); E) A case with mild steatosis demonstrating numerous brown-stained CD163-positive cells in the hepatic lobule, with scant positive cells in the portal tract (lower right) (anti-CD163 200×); F) High power view demonstrating more numerous macrophages with brown staining in the hepatic lobule as compared to those in the portal tract (upper left) (anti-CD163 400×)
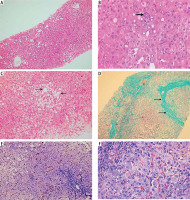
The results of CD163 immunohistochemical staining of liver specimens from patients with MASLD and the distribution of CD163-positive cells in hepatic lobules and portal tracts are presented in Table 3 and Figure 2E, F. The mean count of CD163-positive cells in hepatic lobules (HL) was significantly higher in patients with NAS ≥ 5 than in patients with NAS < 5 (p < 0.001). The mean count of CD163-positive cells in portal tracts (PT) was significantly higher in patients with NAS ≥ 5 than in patients with NAS < 5 (p < 0.001). The total intrahepatic CD163-positive cell count (HL + PT) was significantly higher in patients with NAS ≥ 5 than in patients with NAS < 5 (p < 0.001). The hepatic lobule/portal tract CD163-positive cell count ratio (HL/PT) was significantly lower in patients with NAS ≥ 5 than in patients with NAS < 5 (p < 0.001) (Table 3).
Table 3
Frequency of intrahepatic CD163-positive cells in patients with metabolic dysfunction-associated steatotic liver disease (MASLD) (n = 30) and statistical comparisons between patients with NAS < 5 and NAS ≥ 5
| Parameter | MASLD | NAS | Test of sig. | p | |
|---|---|---|---|---|---|
| n = 30 | < 5 (n = 16) | ≥ 5 (n = 14) | |||
| CD163+ cells in HL | |||||
| Mean ±SD | 112.52 ±30.56 | 93.51 ±22.2 | 134.24 ±23.7 | t = 4.859* | < 0.001* |
| Median (min.-max.) | 112.15 (50-189.6) | 98.05 (50-119.7) | 135.8 (100.8-189.6) | ||
| CD163+ cells/PT | |||||
| Mean ±SD | 8.48 ±3.82 | 5.7 ±2.2 | 11.65 ±2.58 | t = 6.821* | < 0.001* |
| Median (min.-max.) | 7.9 (1.5-15.8) | 6.35 (1.5-8.3) | 11.6 (6.8-15.8) | ||
| CD163+ count (HL+PT) | |||||
| Mean ±SD | 120.99 ±33.9 | 99.21 ±24.01 | 145.89 ±25.44 | t = 5.168* | < 0.001* |
| Median (min.-max.) | 120.65 (51.5-205.4) | 103.8 (51.5-127.8) | 147.7 (107.6-205.4) | ||
| CD163 count ratio (HL/PT) | |||||
| Mean ±SD | 15.47 ±6.01 | 18.66 ±6.55 | 11.81 ±2.01 | U = 26.0* | < 0.001* |
| Median (min.-max.) | 14.49 (7.61-33.33) | 16.27 (10.94-33.33) | 11.45 (7.61-15.02) | ||
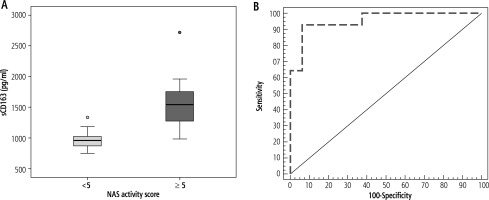
The median sCD163 level was significantly higher in patients with NAS ≥ 5 compared to those with NAS < 5 (p < 0.001) (Table 1, Fig. 3A). ROC curve analysis showed that the sensitivity and specificity of sCD163 level in discriminating patients with NAS < 5 from those with NAS ≥ 5 were 92.86 and 93.75, respectively, at a cut-off value of 1182.4 pg/ml (AUC = 0.955) and the PPV and NPV were 92.9 and 93.7 respectively (Fig. 3B).
Fig. 3
A) Comparison between patients with non-alcoholic fatty liver disease activity score (NAS) > 5 and NAS < 5 according to sCD163, B) ROC curve for sCD163 to discriminate NAS > 5 from NAS < 5
Body mass index (BMI), waist circumference, TG, and HOMA-IR levels were significantly higher in patients with NAS ≥ 5 than those with NAS < 5. The levels of Hb, WBCs, platelet count, ALT, AST, ALP, serum albumin, serum bilirubin, prothrombin activity, total cholesterol, LDL, and HDL showed no difference between patients with NAS < 5 and those with NAS ≥ 5 (Table 1).
Total CD163-positive cell count and sCD163 level were positively correlated (r = 0.999, p < 0.001) (Fig. 4A), sCD163 level showed a positive correlation with NAS (r = 0.873, p < 0.001) (Fig. 4B), and total CD163-positive cell count showed a positive correlation with NAS (r = 0.854, p < 0.001) (Fig. 4C, Table 4).
Table 4
Correlation between sCD163 and intrahepatic total CD163-positive cell count with different parameters in MASLD group (n = 30)
| Parameter | sCD163 (pg/ml) | Intrahepatic total CD163-positive cell count | ||
|---|---|---|---|---|
| rs | p | r | p | |
| Total CD163-positive cell count | 0.999* | < 0.001* | ||
| NAS | 0.873* | < 0.001* | 0.854* | < 0.001* |
| Steatosis | 0.746* | < 0.001* | 0.715* | < 0.001* |
| Ballooning | 0.724* | < 0.001* | 0.699* | < 0.001* |
| Lobular inflammation | 0.575* | 0.001* | 0.550* | 0.002* |
| Fibrosis stage | 0.262 | 0.162 | 0.262 | 0.162 |
| Waist circumference (cm) | 0.776* | < 0.001* | 0.736* | < 0.001* |
| BMI (kg/m2) | 0.957* | < 0.001* | 0.905* | < 0.001* |
| Serum triglycerides (mg/dl) | 0.999* | < 0.001* | 0.918* | < 0.001* |
| HOMA-IR | 0.998* | < 0.001* | 0.976* | < 0.001* |
| APRI | –0.211 | 0.264 | –0.143 | 0.450 |
| FIB-4 | 0.012 | 0.948 | 0.025 | 0.896 |
| NFS | 0.329 | 0.076 | 0.405* | 0.026* |
| BARD score | 0.271 | 0.147 | 0.284 | 0.128 |
Fig. 4
A) Correlation between sCD163 and total intrahepatic CD163 count in metabolic associated steatotic liver disease (MASLD) group; B) Correlation between sCD163 and non-alcoholic fatty liver disease activity score (NAS) in MASLD group; C) Correlation between total intrahepatic CD163 and nonalcoholic fatty liver disease activity score (NAS) in the MASLD group
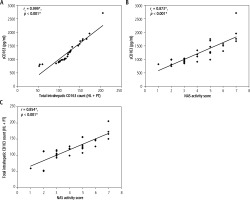
Total CD163-positive cell count and sCD163 level showed positive correlations with steatosis grade, lobular inflammation, and ballooning, but not with fibrosis stage (Table 4).
Total CD163-positive cell count and sCD163 level showed positive correlations with BMI, waist circumference, TG, and HOMA-IR (Table 4).
APRI score, FIB-4 index, NFS, and BARD score in patients with MASLD showed no significant difference between patients with NAS ≥ 5 and those with NAS < 5 (Table 5). Intrahepatic total CD163-positive cell count was positively correlated with NFS (r = 0.405, p < 0.026), but no correlation was found between total CD163-positive cell count or sCD163 and APRI, FIB4 index, or BARD score (Table 4).
Table 5
APRI, FIB-4 index, NFS, and BARD score in patients with MASLD (n = 30) and statistical comparisons between patients with NAS < 5 and NAS ≥ 5
Discussion
This study showed a significantly higher level of sCD163 among patients with MASLD compared with healthy controls and positive immunostaining of CD163-positive macrophages in hepatic lobules and the portal tract in liver biopsy of MASLD patients, suggesting increased expression of CD163-positive macrophages in MASLD patients and enhanced shedding of sCD163 as a response to the inflammatory macrophage activation [25]. Intrahepatic total CD163-positive cell count was positively correlated with serum sCD163 level, suggesting that sCD163 is a marker of KC activation. Moreover, both were correlated with NAS with more significant increases of sCD163 and intrahepatic total CD163-positive cell count in patients with NAS ≥ 5, suggesting that early hepatic lipid buildup may be associated with KC activation and inflammation, even before it is histologically evident and macrophage activation was ongoing throughout all stages of MASLD [16].
During MASLD progression, KCs are activated by free fatty acids, lipotoxic lipid products, and gut-derived lipopolysaccharides that activate the Toll-like receptors (TLR4) and secrete multiple cytokines such as TNF-α, IL-6 and IL-1β [26]. TNF-α and IL-1β induce more lipid accumulation by decreasing peroxisome proliferator-activated receptor α (PPAR-α) transactivating activity, which, in turn, affects fatty acid oxidation and induces lipogenesis [7]. In the progression from MASLD to MASH, hepatocytes undergoing necrosis release danger-associated molecular patterns such as high mobility group box-1 that binds to TLR4 and stimulates TNF-α release in KCs and extracellular vehicles as apoptotic bodies which are engulfed by KCs, resulting in the production of TNF-related apoptosis-inducing ligand leading to hepatocyte death [27].
In agreement with our results, Hegazy et al. revealed that NAFLD patients had higher levels of sCD163 than controls, and CD163 had a positive correlation with NAS [28]. In line with that, Rosso et al. found that both sCD163 and hepatic CD163 were related to the degree of steatosis in the liver biopsy [15]. Moreover, De Vito et al. found that hepatic CD163-positive cell counts were elevated in liver biopsies of children with NAFLD and were significantly elevated in children with NAS ≥ 5 [24]. Also, Kazankov et al. reported that patients with steatohepatitis had higher levels of CD163-positive macrophages, and it was associated with sCD163 [25]. In contrast with our results, Skytthe et al. found that hepatic expression of CD163 had a negative correlation with sCD163 [29]. It could be explained by disease progression, where CD163-positive KCs were depleted and replaced by short-lived monocyte-derived macrophages [30].
The present study showed an increase in BMI and waist circumference in patients with MASLD compared to healthy controls, and both showed a positive correlation with intrahepatic total CD163-positive cell count and sCD163 levels, suggesting an association between adiposity and macrophage activation. Moreover, adipose tissue macrophages probably contribute with KCs to serum sCD163 levels [15]. Obesity appears to contribute to the incidence of steatosis and its progression to steatohepatitis; it is associated with low-grade and chronic inflammation, with higher levels of activated macrophages in the adipose tissue [31]. Besides liver macrophages, activated macrophages in the adipose tissue may add to the development of MASH by inducing the production and secretion of various adipocytokines [32]. This study found a significant increase in HOMA-IR in patients with MASLD compared to healthy controls and showed a positive correlation with intrahepatic total CD163-positive cell count and sCD163 levels, and levels of HOMA-IR were even higher in patients with NAS ≥ 5, which can be explained by obesity, which is linked to the development of insulin resistance and MASLD progression. Insulin resistance makes adipose tissue resistant to the antilipolytic effect of insulin, allowing TG to be broken down and increasing the overflow of free fatty acids to the liver, promoting steatosis and lipotoxicity, ending in cellular dysfunction and apoptosis, an essential feature of MASH [33].
This study showed a significant increase in levels of TG in patients with MASLD compared to healthy controls and showed positive correlations with sCD163 levels and intrahepatic total CD163-positive cell count. That could be explained by the excessive release of free fatty acids from adipose tissue, due to the effect of insulin resistance, which act as substrates for the excess synthesis of hepatic very-low-density lipoprotein, and interference in triglyceride-rich lipoproteins’ clearance [34]. In line with this, Schwenger et al. reported that compared to healthy controls, patients with nonalcoholic steatohepatitis had higher TGs, HOMA-IR, and BMI [35]. Also, higher TG levels and waist circumference were found in children with NAS ≥ 5, as reported by De Vito et al. [24]. In agreement with our results, a study by Hegazy et al. showed a significant correlation between the level of CD163 and BMI [28].
Alanine aminotransferases reflects hepatocyte necrosis, which increases during lipoapoptosis with disease progression [36]. It might explain the significant increase of serum ALT level in MASLD patients compared to controls in our study, but it did not correlate with sCD163 level. In line with our findings, a study by Schwenger et al. found a higher level of ALT in patients with steatosis than in healthy subjects [35]. Also, Hegazy et al. reported no significant correlation between CD163 levels and ALT [28].
Our study showed no relation between APRI score, FIB-4 index, NFS, BARD score, and sCD163 in patients with MASLD with only a correlation between intrahepatic total CD163-positive cell count and NFS, which may be due to a low degree of fibrosis in our patients, and normal laboratory parameters of our patients also may contribute to this result. In line with that, Soresi et al. found that using serum markers to evaluate the presence of fibrosis did not yield reliable results [37]. In disagreement with our study, Önnerhag et al. reported that liver-related complications can be predicted by non-invasive fibrosis scoring systems [38].






