Purpose
Cervical cancer is still one of the leading causes of deaths in women worldwide, especially in developing countries [1]. Radiation therapy (RT) is the main treatment method for cervical cancer around the globe, especially in developing countries, as most patients present with locally advanced stages. Standard radiotherapeutic treatment consists of external beam radiation therapy (EBRT) to whole pelvis, followed by intra-cavitary brachytherapy (ICBT). Different dose-rate systems are employed for ICBT, including low-dose-rate (LDR), high-dose-rate (HDR), and pulsed-dose-rate (PDR). Although LDR is the most practiced over several decades, HDR is the most popular modality, both in developed [2, 3] and developing countries [4, 5]. Though declining over years, several centers, especially in developing countries, continue to use LDR, benefiting a large population of cervical cancer patients. Such centers prefer LDR mainly because of cost reasons, as cesium-137 (137Cs) radioactive source does not need frequent replacements, unlike HDR brachytherapy due to its longer half-life. In India, there are 250 HDR and 67 LDR brachytherapy units [4]. According to Gynecologic Cancer Intergroup (GCIG) survey [2], considerable number of centers in USA (29%) still use LDR and PDR (LDR, 18%; PDR, 11%) brachytherapy for cervical cancer. American Brachytherapy Society (ABS) issued practice guidelines [6] for LDR- and PDR-based ICBT utilization in cervical cancer.
Pulsed-dose-rate is the newest of all dose-rate systems, and the literature is extremely scarce. In PDR, multiple hourly HDR pulses, lasting for few minutes, are delivered [7]. Typically, the overall treatment time and dose are the same as corresponding LDR regimen. PDR has several advantages over LDR, such as improved dosimetry, better patient care, and higher therapeutic ratio [8, 9]. It is believed to be superior to LDR and HDR, since it combines radiobiological and geometrical merits of both. However, there is no study supporting its supremacy. PDR is gaining popularity in some parts of the world [3], and results of its utilization are encouraging [8, 9]. According to the survey conducted in Europe, PDR is the second most popular dose-rate system (available in 17% centers) after HDR (63% centers) [3].
In the absence of randomized control trials, it is justifiable to compare clinical data relating to LDR-, HDR-, and PDR-ICBT to assess the best dose-rate system.
Our institution is the premier medical institute in India, and the first to have the only available PDR unit in the country. Till the year 2003, we had one LDR remote afterloading unit, and later added one HDR and one PDR unit. In 2003, our brachytherapy practice was changed into PDR and HDR regimens, and LDR practice was completely abandoned in the year 2004. Even though initially there was no specific policy for selecting patients for HDR or PDR treatments, later there were offered to patients from distant areas, for whom frequent traveling for multiple HDR fractions was difficult. In this study, we analyzed our institutional clinical data to compare LDR-, HDR-, and PDR-ICBT for cervical cancer in order to determine the best dose-rate system.
Material and methods
Study design
This retrospective study included 613 cervical cancer patients’ data, who were treated with whole pelvis EBRT, followed by either LDR- (271 patients), HDR- (259 pa- tients), or PDR-ICBT (83 patients). LDR group patients were treated before the year 2003 (from 1992 onwards). In 2004, our institute decommissioned LDR facility and shifted to HDR and PDR practice. Case records of all these patients were retrieved to obtain data regarding patients’ demography, clinical details, diagnosis, treatment given, survival, and long-term morbidity. Exclusion criteria were as follows: 1. Patients with not intact uterus; 2. Patients who could not complete the prescribed treatment course of EBRT or ICBT; 3. Patients who received pelvic EBRT in an outside hospital, and later received ICBT at our center; 4. Patients with para-aortic (PA) node metastases; and 5. Patients not treated with conventional ICBT applicators, such as tandem-ovoid, tandem-ring, etc.
Treatment
The initial pre-treatment workup consisted of thorough clinical examination by a team comprising of gynecologists and radiation oncologists. Each patient was subjected to routine hematological and imaging investigations, including plain chest radiograph, abdomen-pelvic ultrasonography, or CT imaging, and staged according to the existing International Federation of Gynecology and Obstetrics (FIGO) system [10].
EBRT: All patients were treated with whole pelvis EBRT followed by ICBT. EBRT was delivered by either linear accelerator (15 MV) or cobalt-60 unit using two AP-PA or four-field box technique (isocentric technique). IMRT or VMAT was not used. A dose of 50 Gy with conventional fractionation (1.8-2.0 Gy per fraction, 5 fractions per week) was prescribed at midplane/isocenter. For AP-PA portals, superior border was kept at L4-L5 junction, inferior border at 3 cm below the lower extent of disease, at the bottom of obturator foramina, and lateral border at 2 cm lateral to bony pelvic brim. For lateral portals, superior and inferior borders were the same as in AP-PA portals, anterior border was placed anterior to pubic symphysis, and posterior border at sacral hollow or S2-S3 level depending on the extent of disease.
Chemotherapy: Since our institute did not adopt concurrent chemotherapy practice till 2003, LDR patients were treated without chemotherapy. For patients in HDR and PDR group, weekly chemotherapy (cisplatin, 40 mg/m2) was administered every week during the course of EBRT. Patients aged more than 70 years, with deranged kidney function tests and refusal for chemotherapy were not considered for chemotherapy treatment. Blood transfusion was performed if the hemoglobin level fell below 10.0 g/dl. After EBRT, patients were assessed for standard ICBT application. ICBT was performed within one week of completion of EBRT. All ICBT treatment plans were based on the TG-43 water-based model.
LDR-ICBT: Under spinal or general anesthesia, Fletcher-Suit applicator was inserted using small, medium, or large ovoids (according to vaginal space). Adequate vaginal packing was done to minimize dose to the bladder and rectum. Orthogonal X-ray images were obtained to verify placement of the applicator, and to calculate dosimetry using Nucletron version 11.43 system planning. The dose was prescribed at point A, defined as 2 cm above and 2 cm lateral to the cervical os, or flange along the plane of the tandem. Bladder and rectal points were defined as per the ICRU Report 38 [11]. Treatment was delivered with 137Cs remote afterloading brachytherapy unit (Selectron). Dose-rate at point A ranged from 160 to 190 cGy per hour. Since this dose-rate was higher than in classical LDR, a dose reduction factor of 15% was applied for dose prescription [12]. Therefore, a dose of 30 Gy was prescribed at point A in single-session. After completion of ICRT, the applicator was removed, and vaginal packing was done if necessary.
HDR-ICBT: The treatment was carried out in 3 fractions using once-a-week schedule. For each session, the Fletcher-Suit applicator was inserted under conscious sedation, or general/spinal anesthesia if needed. Trans-rectal ultrasound was applied if there was difficulty in inserting the central tandem. Computed tomography (CT)-based, point-based planning (Plato planning system, version 14.1) was done for every fraction, with a prescription dose of 7 Gy at point A. Multiple points were marked along the posterior surface of the Foley’s bulb visible on sagittal CT image, and the posterior most point or the point receiving the highest dose was labeled as the bladder reference point (Bladderref point). Similarly, multiple points were placed along the anterior most surface of the rectal marker tube (visible on sagittal CT image), starting from ano-rectal junction up to recto-sigmoid junction. The closest point to the central tandem or the point receiving the highest dose was labeled as the rectal reference point (Rectalref point). Multiple active dwell positions were selected in the central tandem and ovoids at an interval of 2.5-5.0 mm to create a pear-shaped dose distribution with minimal dose to Bladderref point and Rectalref point. The treatment was delivered using MicroSelectron HDR remote afterloading unit, with iridium-192 (192Ir) source and initial activity of 37 × 1010 Bq.
PDR-ICBT: The procedure of applicator insertion and treatment planning was the same as in HDR-ICBT, except that the treatment was delivered in a single-session. Using the same TPS as in HDR planning, a dose of 27 Gy was prescribed at point A, employing 39 pulses (each pulse of 70 cGy delivered every hour). The treatment was delivered 24 hours/day using PDR remote afterloading unit (MicroSelectron, Nucletron), with 192Ir source and initial activity of 3.7 × 1010 Bq. Nursing care was provided in between the scheduled pulses.
Follow-up
Patients were followed up every 3 months till 2 years, and every 6 months thereafter. At every visit, clinical examination was performed and, if required, computed tomography or magnetic resonance imaging (MRI) scans to assess the disease status and toxicity.
Statistical analysis
Statistical analysis was performed using SPSS version 16. The endpoints of the study were overall survival (OS) and long-term toxicity. OS was defined as the period from the date of histopathological diagnosis to the date of death or last follow-up. OS was determined by the Kaplan-Meier method [13]. Patients who were lost to follow-up after a certain period were censored at that point of time for survival analysis. Survival curves were compared with log-rank test [14], and a p-value of < 0.05 was considered significant. Late toxicity was assessed according to the Radiation Therapy Oncology Group (RTOG) criteria [15]. Only grade III/IV toxicity was considered for comparison between different groups.
Results
Patients’ demographics are shown in Table 1. All three groups were comparable (p-values not significant). The median age of the entire cohort of patients was 49 years (range, 24-78 years). Overall, the majority of patients (329/613) had stage III disease at presentation. Stage II was the commonest stage in the LDR group, while stage III was the most common in the HDR and PDR groups. Squamous cell carcinoma was the typical histological type across all the three groups. The median size of the primary tumor was 4 cm (range, 3-9 cm). The median follow-up was shorter in the PDR group (30 months) compared with the other 2 groups (LDR, 60 months; and HDR, 52 months).
Table 1
Patient and tumor characteristics
Table 2 shows the details of the treatment received by the patients of three groups. The overall treatment duration for EBRT plus ICBT was shorter in the LDR group compared with the HDR and PDR groups (LDR, 47; HDR, 57; and PDR, 54 days). The LDR group did not receive any chemotherapy, as concurrent chemoradiotherapy (CCRT) was not a standard treatment at that time. The proportion of patients receiving chemotherapy in the HDR and PDR groups was comparable (74% vs. 73%), with a median of 4 cycles in each group. The median EBRT dose was similar across the three groups. The mean ICBT dose to Rectalref point (% of point A) was below 70% in each group (Table 2). The mean ICBT dose to Bladderref point (% of point A) was comparable in each group (~70%).
Table 2
Details of treatment
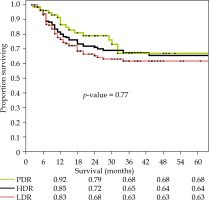
Loco-regional control (LRC) rate in the LDR, HDR, and PDR groups was 74%, 75%, and 77%, respectively (p-value = 0.80) (Table 3). As shown in Figure 1, there was no statistically significant difference in OS at 5 years in the three groups (LDR, HDR, PDR: 63%, 64%, 68%, respectively; p = 0.77). Since the number of stage I patients was very small in all groups (Table 1), subset analysis for stage II and stage III was performed. The analysis revealed no significant difference in OS in stage II, but stage III patients had significant inferior 5-year OS in the LDR group as compared with the HDR group (p = 0.038) and PDR group (p = 0.036) (Table 3). There was no difference in 5-year OS in the entire cohort of patients treated with CCRT and RT alone (71% and 66%, p = 0.42). Other factors, such as pre-treatment hemoglobin level, age, and overall treatment time had no significant correlation with 5-year OS.
Table 3
Loco-regional control, survival, and toxicity rates in three groups
Fig. 1
Kaplan-Meier curve showing 5-year overall survival for low-dose-rate (LDR), high-dose-rate (HDR), and pulsed-dose-rate (PDR)
The overall late grade ≥ 3 toxicity was observed in 33 LDR patients, 21 HDR patients, and 8 PDR patients, and the difference was statistically insignificant (p = 0.36). Late grade ≥ 3 bladder toxicity rate was comparable in the three groups (LDR, 4%; HDR, 3%; PDR, 4%, p = 0.96). Similarly, late rectal grade ≥ 3 toxicity rate was comparable in the three groups (LDR, 8%; HDR, 5%; PDR, 5%, p = 0.42). None of the patients underwent surgical intervention (colostomy or bladder diversion) for management of toxicity. Bladder toxicity was managed by continuous bladder irrigation with saline or alum, or by instilling formalin (2%) under general or spinal anesthesia. Rectal toxicity was handled by steroid enemas, argon plasma coagulation, and intra-rectal formalin (4%) application.
Discussion
Since ICBT is the most common brachytherapy procedure practiced worldwide, the question “Which is the best dose rate system?” concerns a large number of brachytherapists engaged in the management of cervical cancer. Each dose-rate system has its own advantages and disadvantages. Various studies have compared clinical data with different dose-rate systems (mostly LDR and HDR), and published literature have showed equivalent results with LDR and HDR in terms of survival and toxicity rates [16]. PDR has been rarely compared with LDR and HDR. Despite the individual centers preference for a particular dose-rate system, all the three systems are in clinical use for the treatment of cervical cancer. To the best of our knowledge, the present study is the very first to compare all the three existing dose-rate systems, and will be useful addition to the literature.
The results of our study reveal comparable LRC rates and 5-year OS rates among all the three groups, with no statistically significant differences (p = 0.77) (Table 3). Subset analysis showed stage as the significant prognostic factor in all the groups. We noticed comparable LRC and 5-year OS for stage II patients in all the 3 groups (Table 3). For stage III, though LRC was comparable in all the groups, the 5-year OS was significantly inferior for the LDR group. Most published literature demonstrates similar clinical outcomes regarding LDR and HDR brachytherapy in all stages [16], but few studies report contrasting clinical outcomes for stage III. Two previously published reports [17, 18] found inferior outcomes for higher stage patients (FIGO stage III vs. stage IB/II) treated with HDR, while one study [19] reported better results with HDR in stage III patients. Petereit et al. [17] retrospectively compared the results of 91 patients treated with LDR-ICBT and 173 treated with HDR-ICBT. They observed comparable results for stage IB/II patients treated with either HDR- or LDR-ICBT, but inferior 3-year survival (33% vs. 58%, p = 0.004) and poorer 3-year pelvic control (44% vs. 75%, p = 0.002) for stage IIIB patients treated with HDR brachytherapy. Ferrigno et al. [18] compared the results of 190 patients treated with LDR and 118 patients treated with HDR. They observed that for clinical stage III, OS and disease-free survival (DFS) at 5 years were better in the LDR group than in the HDR group (46% vs. 36%, p = 0.04, and 49% vs. 37%, p = 0.03, respectively). In contrast, Kucera et al. [19] reported better results with HDR compared with LDR brachytherapy (53.8% vs. 37.3%, p < 0.01) in stage III patients. Such wide variation in the results with different dose rates is possibly because of the different prognostic factors involved, such as tumor volume, extent of parametrial invasion (bilateral or unilateral), presence of hydronephrosis, nodal metastasis, and extent of vaginal involvement. These factors should be considered when analyzing the results. Due to retrospective nature of the present study, we could not stratify patients according to the above-mentioned factors.
Additionally, we could not find any study in the literature comparing the results of PDR brachytherapy and other dose-rate systems for stage III patients. Inferior 5-year OS rate in the LDR group in stage III in our study is probably attributable to sub-optimal coverage of the tumor. As LDR patients were treated before the year 2003, ICBT planning was X-ray-based, and no chemotherapy was administered in practice. LDR-ICBT was delivered in a single-session after EBRT. Patients showing poor tumor shrinkage and large residual growth might have had sub-optimal dosimetric coverage. On the other hand, HDR-ICBT was delivered in multiple weekly fractions and tumor shrinkage continued in between the fractions, providing better probability of improved dosimetric coverage in subsequent ICBT fractions. HDR- and PDR-ICBT planning was CT-based, and therefore had better dosimetry than X-ray-based planning in the LDR group.
Our results cannot be compared with other studies, since there is none in the literature. Further, the literature is extremely scarce on PDR brachytherapy. There is only one randomized study [20] available in the literature comparing PDR with HDR. Kumar et al. [20] conducted a randomized trial comparing HDR- and PDR-ICBT in locally advanced cervical cancer. They reported comparable 4-year DFS (67.1% vs. 71.8%, p = 0.195) and OS (77% vs. 75%, p = 0.322) in HDR- and PDR-ICBT, respectively. There was no statistically significant difference in late rectal toxicities grade ≥ 2 (16.7% vs. 21.1%, p = 1.000), late bladder toxicities grade ≥ 2 (10.5% vs. 0.0%, p = 0.486), and late vaginal toxicities grade ≥ 2 (15.8% vs. 5.6%, p = 0.604) for HDR and PDR, respectively.
The overall late toxicity, and bladder and rectal toxicity rates were comparable in the three groups in our study (Table 3), and are consistent with the literature [9, 21, 22]. Due to large dose per fraction, HDR brachytherapy may have a hypothetical risk of higher late toxicity. This biological disadvantage can be overcome by appropriate fractionation and total dose. Moreover, dose gradient related to HDR brachytherapy reduces the risk of late toxicity. The literature [21, 22] show equivalent late toxicity rates in both LDR and HDR brachytherapy. Published data on PDR brachytherapy reported severe late toxicity rate of 3-9% [9], which is comparable with HDR and LDR brachytherapy.
Addition of concurrent chemotherapy to RT has been shown to provide significant survival benefit [23]. Since the LDR group patents in our series did not receive concurrent chemotherapy, it is worth arguing whether lack of chemotherapy affected the results. The subset analysis did not show any significant impact on 5-year OS in the HDR and PDR groups treated with concurrent chemoradiotherapy. Another possible explanation is that majority of our patients had locally advanced disease, where addition of chemotherapy showed very small (3%) survival advantage [23]. In randomized trials [24, 25], no survival benefit of CCRT have been proven.
Most PDR series [9] used ICBT dose ranging from 15-40 Gy, with hourly pulse-dose of about 0.5 Gy. ABS [6] recommends 35-45 Gy in PDR-ICBT after 45 Gy of EBRT. Here, we used relatively lower dose (27 Gy), because our EBRT dose was 50 Gy and the hourly pulse-dose was 0.7 Gy.
In the current study, there are some limitations, including its retrospective nature, prolonged duration, and no chemotherapy for LDR patients. At the same time, the study has several strong points, such as adequate sample size, all patients received EBRT and ICBT at one institute, uniform EBRT practice across all the groups, and implementing learning.
Conclusions
The present study demonstrated comparable local control, 5-year OS, and severe late toxicity rates in the analyzing groups of patients. Lower OS rates were observed in the LDR group in stage III patients as compared with the HDR and PDR groups. Learning experience from LDR practice refined our HDR- and PDR-ICBT practice. This could be one of the possible reasons for improved results with HDR-ICBT and PDR-ICBT. Therefore, it is suggested that LDR users should be careful while treating stage III patients, although they can continue using LDR for the rest of stages. Despite small sample size, PDR yielded marginally higher LRC and OS rates as compared with LDR and HDR, and therefore needs to be further researched with bigger sample size to examine its superiority.



