Brachial plexus block is the most popular regional technique for surgery of the upper limbs from the shoulder to the finger. Supraclavicular and infraclavicular are the two widely used approaches that provide dense anaesthesia of the whole upper limb, including the arm, forearm and hands. These two approaches have their advantages as well as concerns [1, 2].
Infraclavicular brachial plexus block provides better nerve blockade with lower incidence of ulnar sparing compared to supraclavicular block. It is conventionally done at the lateral infraclavicular fossa (LIF), where the cords lie deeper to the pectoralis muscles around the second part of the axillary artery. The steep angle needed to reach the deeply placed cords makes it difficult to discern the needle tip and the needle direction. This makes it difficult to reach the cords arranged around the artery without risking vascular puncture. Moreover, there is inconsistency in the cord position relative to the axillary artery at the LIF [3–6].
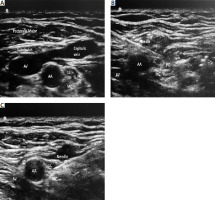
The costoclavicular approach is a relatively new approach of infraclavicular brachial plexus block and blends the block characteristics of the conventional infraclavicular approach with the procedural ease of the supraclavicular block. It can be consi-dered as an infraclavicular approach for the supraclavicular divisions and cords. In contrast to LIF, the plexus is superficial at the costoclavicular space and is consistently clustered in a triangular arrangement on the lateral side of the artery in the transverse view (Figure 1). This enables easier needle visualisation and achieves a reliable and consistent block with a single injection between the cords. Compared to the supraclavicular block, the brachial plexus is located within a “tunnelled muscular canal” bounded anteriorly by the subclavius and clavicular head of the pectoralis major and posteriorly by the anterior chest wall. This makes the costoclavicular approach ideal for catheter placements. Since it is an infraclavicular approach, there is also a decreased risk of phrenic palsy [8–14].
FIGURE 1
A) Normal anatomy for costoclavicular block. B) Medial-to-lateral approach. C) Lateral-to-medial approach. AA – axillary artery, AV – axillary vein, LC – lateral cord, MC – medial cord, PC – posterior cord
Conventionally, a lateral-to-median approach is used for costoclavicular block (CCB) to target the cords located lateral to the axillary artery. However, there are a few concerns with this approach. The presence of the coracoid process in the procedural site cause difficulty in manoeuvring the block needle, and the block direction towards the vascular structures and pleura can cause inadvertent mechanical injury to those structures [14–16].
We hypothesise that a medial-to-lateral approach might be a better alternative for the CCB owing to the above disadvantages. We assume that the needle direction away from the pleura and axillary vessels decreases the risk of injury whereas the absence of bony structures enables faster block performance.
We compared the block characteristics including imaging time, needling time, performance time, block success rate, procedural complications, procedural difficulty and patient outcomes such as patient satisfaction between the two approaches.
Methods
The clinical trial commenced only after receiving ethical approval from the Institutional Ethics Committee, AIIMS – New Delhi, India (IECPG-720/19.12.2019, RT-03/30.01.2020) and subsequent regi-stration in the clinical trial registry, India (CTRI/2020/03/024205) according to the Declaration of Helsinki. Inclusion criteria encompassed patients aged 18 to 70, categorized under physical status ASA (American Society of Anesthesiologists) I and II, with a body mass index (BMI) falling within the range of 18 to 35. The study focused on individuals undergoing elbow, forearm, wrist, or hand surgery at our orthopaedic surgical theatre. Exclusion criteria comprised patients who declined participation, those with coagulopathy, sepsis, on anticoagulants, having allergies to local anaesthetics, experiencing neuropathies, or presenting with local infections.
Randomisation and blinding
All the patients recruited for the study were randomly allocated to two groups using a computer-generated sequence of random numbers, employing a sealed envelope technique. They all received CCB via either a medial-to-lateral (Group M) or a lateral-to-medial (Group L) approach. It was not feasible to blind the primary investigator, who assessed the performance times, or the anaesthesiologists, who performed the block and rated the difficulty. The remaining data were systematically collected by an independent blinded observer uninvolved in the procedure. Both the patient and the statistician responsible for data assessment remained unaware of the group allocation.
Intervention
The study details, risks, and benefits were explained to the patient, and written informed consent was obtained a day before surgery. After arrival in the induction room, an 18G wide bore IV cannula was inserted and intravenous premedication (fentanyl 0.25 μg kg–1 and midazolam 1 mg) was admi-nistered to all patients. Anaesthesiologists who had experience with at least twenty previous ultrasound-guided brachial plexus blocks performed the block. Patients were placed supine with the relevant limb in 90-degree abduction. A high-frequency linear transducer (SonoSite S-Nerve, Fujifilm Sonosite Inc, WA, USA) was placed immediately below and parallel to the middle one-third of the clavicle. The transducer was tilted slightly cephalad to visualise the costoclavicular space. Pre-block ultrasound scanning was done to assess the anatomy and measure the depth of the cords at the costoclavicular space. Ultrasound images were optimised until all three cords were identified, lateral to the first part of the axillary artery (Figure 1). After that, the patients received CCB via either a medial or lateral approach, depending on their randomly allocated groups.
Group L: The block needle was advanced in a la-teral-to-medial direction for a lateral approach until its tip was in the middle of all three cords, and local anaesthetic (LA) was injected after negative aspiration (Figure 1).
Group M: The needle was inserted from the medial side with the needle tip positioned in the middle of all three cords, and LA was injected after negative aspiration (Figure 1).
A 22G 10-cm block needle (Echoplex+, VYGON, France) and 20 mL of 0.5% bupivacaine were used for all subjects.
Outcome parameters
The anthropometric details of age, sex, weight, height, and BMI were collected and the imaging, needling, and performance times were recorded during the block. Imaging time was defined as the interval between contact of the ultrasound probe with the patient and the acquisition of a satisfactory image. The needling time was defined as the interval between needle insertion and the end of LA injection through the block needle. The performance time equalled the sum of imaging and needling times. The number of needle advancements/manipulations was also recorded. The initial needle insertion counted as the first pass, and any subsequent needle advancement preceded by a retraction of at least 10 mm was counted as an additional pass. After the block, the anaesthesiologist performing the block graded the procedural difficulty as mild (grade 1)/moderate (grade 2)/hard (grade 3).
After LA injection through the block needle, sensory and motor blockade of the musculocutaneous, median, radial, and ulnar nerves was assessed by the observer every 5 minutes (min) to 30 min on a three-point scale (Table 1). The maximum possible composite sensorimotor score was 16 points. We considered the block success and the patient ready for surgery when a minimum composite score of 14 points was achieved, provided the sensory block score was equal or superior to 7 out of 8 points [17]. The block onset time and anaesthesia time were recorded, where block onset time was defined as the time required to obtain a composite score of 14 points and total anaesthesia-related time was defined as the sum of performance and block onset times.
TABLE 1
Scoring to assess sensory and motor block
| Sensory block scoring | |||
|---|---|---|---|
| Score | No block | Analgesia | Sensory |
| Musculocutaneous | 0 | 1 | 2 |
| Radial | 0 | 1 | 2 |
| Ulnar | 0 | 1 | 2 |
| Median | 0 | 1 | 2 |
| Motor block scoring | |||
| Score | No effect | Paresis | Paralysis |
| Musculocutaneous | 0 | 1 | 2 |
| Radial | 0 | 1 | 2 |
| Ulnar | 0 | 1 | 2 |
| Median | 0 | 1 | 2 |
If the composite score were inferior to 14 points after 30 min, the patient would be transferred to the operating room for the start of the surgery. We would not record an onset time or perform supplemental blocks for these subjects. The same observer would record the block success, defined as the surgery being done without the need for gene-ral anaesthesia, rescue blocks, or local anaesthetic agent infiltration by the surgeon.
We assessed diaphragmatic excursion with M-mode ultrasound before and 30 min after performing the block. This was done by a low frequency (1 to 5 MHz) curved array transducer (Sonosite S-Nerve, Fujifilm Sonosite Inc, WA, USA), via the anterior subcostal route, with the liver serving as the acoustic window for the right diaphragm and with the spleen serving as an acoustic window for the left diaphragm. Once a stable M-mode trace was obtained with quiet breathing, the extent of the diaphragmatic excursion was measured as the maximum distance from the beginning of inspiration to peak inspiration using the electronic calliper of the ultrasound system. Diaphragmatic measurements were done thrice, and the average values were recorded.
After the procedure, ultrasound screening was also done to rule out any complications such as pneumothorax or haematoma. Patient satisfaction was also graded by a visual analogue pain score (0 = no pain; 10 = worst imaginable pain). At the end of the surgery, the patients were transferred to the post-anaesthesia care unit for monitoring.
Sample size and statistical analysis
An extensive search across various databases found no comparative studies available. One prior study reported a performance time of approximately 6.7 min using the lateral approach [17]. Based on observations from a smaller-scale study conducted at our institute, indicating a reduced time with the medial approach, we hypothesized a 25% reduction in performance time. For a 95% confidence interval and 80% power and allocating patients in a 1 : 1 ratio to both groups with a dropout rate of 10%, the total sample size was determined to be approximately 56 patients (28 patients per group). Rounded to enhance robustness, we enrolled 60 patients, with 30 in each group.
Data are presented as number (%), mean ± SD, or median (min-max) as appropriate. Baseline charac-teristics were compared between groups using the χ2 test/Fisher’s exact test for categorical traits and the t-test for continuous variables. Normally distributed outcomes were assessed via the t-test for independent samples, while non-normally distributed variables were compared using the Wilcoxon rank sum test. Statistical significance was set at a P-value < 0.05.
Results
We enrolled 62 patients for eligibility, two of whom declined to participate in the study; one patient, after recruitment to Group L, had unfavourable sonoanatomy of the cord for CCB and hence was excluded from the study. Of the remaining 59 patients, 30 were randomised to Group M, and 29 were randomised to Group L. Two patients from Group M were again switched to Group L as the sonographic anatomy made it impossible to perform the block by a medial approach (Figure 2, CONSORT diagram). We conducted statistical analysis by modified intention-to-treat (mITT) analysis and as per protocol analysis. The results from the mITT analysis were summarised.
The demographic and baseline characteristics including age, sex, ASA physical status, anthropometric characteristics such as weight, height and BMI, baseline vitals and depth of plexus were similar between the groups (Table 2).
TABLE 2
Demographic and baseline characters between the groups
The time required for imaging (Group M: 2 min, range 1–6; Group L: 1 min, range 1–5; P = 0.038) and needling (Group M: 9.5 min, range 5–16; Group L: 7 min, range 4–19; P = 0.030) was significantly longer in Group M compared to Group L. Consequently, our primary outcome, the performance time (Group M: 11.9 min, SD = 3.8 vs. Group L: 9.4 min, SD = 4.1; P = 0.018) was also significantly longer in Group M than Group L. Subjective rating of difficulty in performing the CCB with two different approaches revealed significantly greater performer difficulty in patients enrolled in Group M, with the percentage of grade 3 (hard) performer difficulty being 40% vs. 13%; P = 0.032 (Table 3).
TABLE 3
Assessment of outcome parameters between the groups
Other secondary outcomes – block onset time, success rate, and procedure complications – were comparable between the both studied groups. Total anaesthesia time, which also included time for performance, was significantly longer in patients with the medial approach (Group M: median 36 min, range 22–48, Group L: median 31 min, range 19–48; P = 0.019). Compared to the lateral approach, the number of needle advancements was also higher with the medial approach (Group M: median 3 attempts, range 1–6, Group L: median 2 attempts, range 1–4; P = 0.006). We checked for a diaphragmatic excursion before and after the blocks with both approaches. None of the patients in our study developed diaphragmatic palsy (Table 3).
We carried out a subgroup post hoc analysis to assess the effect of depth and increased BMI on the time to perform the block by both approaches. We categorised patients into two groups based on their BMI and depth at the mid-claviculoacromial point. Patients with BMI < 25 were categorized as Group B1 and those with BMI > 25 as Group B2. Forty-three patients (72.8%) had a BMI < 25, and 16 patients (16%) had a BMI > 25. We noted a mean performance time of 9.95 min in patients with BMI < 25 and 12.68 min in patients with BMI > 25, which was statistically significant (P = 0.0243) (Table 4).
TABLE 4
Effect of body mass index (BMI) and depth on performance times with different approaches
Patients with a depth at mid-claviculoacromial point < 3 cm were categorised as Group D1 and patients with a depth > 3 cm were categorised as Group D2. Fourteen patients (23.7%) had a depth < 3 cm, and 45 patients (76.3%) had a depth > 3 cm. We noted a mean performance time of 8.28 min in patients with depth < 3 cm, and 11.44 min in patients with depth > 3 cm, which was statistically significant (P = 0.0123) (Table 4).
Discussion
Costoclavicular brachial plexus block has had a remarkable pace of advancement since the time that Karmakar et al. [10] first published their pioneering anatomical study. It outpaced other infraclavicular brachial plexus block approaches due to the reliable sonoanatomy and better block characteristics. Contemporary studies have shown better outcomes and compared the newer costoclavicular approach with erstwhile supraclavicular and interscalene block. However, alternate approaches to the block at the costoclavicular space are still lacking.
To bridge this lacuna, in this randomised double-blinded trial, we compared the traditional ultrasound-guided costoclavicular brachial plexus block (lateral approach) with a modified approach (medial approach). Nieuwveld et al. [15, 16] have highlighted the difficulty in needling from the lateral to the medial direction due to the coracoid process being an obstacle, especially in the arm abducted position. They have proposed the medial approach, as needle insertion is away from vascular structures and pleura and unhindered by the coracoid process.
We conducted this study with the hypothesis that CCB through the medial approach would result in similar or shorter performance time owing to the absence of bony anatomical structures in the medial aspect affecting the needling. Our study, however, found that the imaging time, needling time, and thus performance time (in min) by mITT analysis were significantly longer with the medial approach (11.9 ± 3.8 min) compared to the lateral approach (9.4 ± 4.1 min). The performer also needed significantly more needle advancements, which led to rating the medial approach as more difficult than the lateral approach (procedure difficulty score; P = 0.032). All the other secondary parameters, i.e. block onset time, block success rate, rescue analgesics, and incidence of diaphragmatic palsy, were similar in both groups.
This significant difference in the groups’ imaging, needling, and performance times could be attributed to the three anatomical and sonoanatomical characteristics encountered while needling in the medial to the lateral direction. First, the axillary vein, which lies medial to the artery with its tributary cephalic vein, could have provided sonological difficulty, and the anaesthesiologist performing the block would have been apprehensive about the vascular structure to avoid accidental vascular puncture. Second, the presence of the septum in the costoclavicular space that separates the superficial lateral cord from the deeper posterior and medial cords makes it easy to navigate the needle to the superior part of the plexus from a medial approach while causing difficulty in accessing the inferior part of the plexus [20, 21]. This warrants multiple needle advancements. Third, some patients had difficult anatomy wherein the medial cord lay dorsal to the artery, making it difficult with the medial approach.
The lateral approach needed less time and fewer manipulations, and a single injection in the junction of the cords was sufficient most of the time. Plexus is located lateral to the artery, making it obvious and readily accessible with a lateral approach. It is also easy to target the whole plexus in a single attempt. The block success rate was similar in both groups. This may be because all three plexus cords are clustered tightly in a consistent relationship to the axillary artery and each other [10–12, 19]. These three cords are arranged inside a common collagen fibrous para-neural sheath, enclosing the axillary vessels to form a common neurovascular sheath [20]. The original approach, therefore, advocated that a single injection between the cords should be sufficient to achieve the block.
Procedural complications were noted in two patients (6.67%) in Group M and one (3.45%) in Group L. The two patients in Group M had blood aspiration during the procedure, with one patient developing subclavicular hematoma and the other having an uneventful post-operative course. All patients were discharged and went home by post-operative day 1 without any adverse events.
There is always a risk of phrenic nerve palsy with different approaches to the brachial plexus: interscalene (100%), supraclavicular (30–60%), infraclavicular (0–3%), and minimal to no risk with the axil-lary approach [7–9, 24]. A search in the literature revealed a lower but varying incidence (0–5%) of diaphragmatic palsy with a CCB [7, 8, 24]. In our study none of our patients developed diaphragmatic dysfunction after the block. This might be explained by the lower volume of local anaesthetic agents injected in our study and the distant location of the costoclavicular space from the phrenic nerve.
Obesity and depth of target nerves also have a significant effect on the performance of the block. We conducted a post-hoc analysis to assess the effect of BMI and depth on the performance time with both medial and lateral approaches. We stratified the study population into two groups based on two factors (BMI and depth of the cords) and analysed the results. The mean performance time was 9.95 min in patients with BMI < 25 and 12.68 min in patients with BMI > 25, which was statistically significant (P = 0.024). Similarly, the mean performance time was 8.28 min in patients with a depth < 3 cm and 11.44 min in patients with a depth > 3 cm, which was statistically significant (P = 0.012). No other studies have correlated these two variables. We assume that the greater the BMI and depth at the midclaviculoacromial point, the greater will be the performance time due to greater needling angulation associated with greater depth.
LimitationS
Our study had several limitations. It is a single-centre trial with a limited sample size. The primary investigator could not be blinded to the primary outcome. Our analysis of BMI and depth was not comprehensive and was only a post hoc analysis for the limited sample size.
Conclusions
Our study has formally assessed the feasibility and patient-specific block dynamics of the medial approach of the CCB and compared it with the lateral approach of the CCB. The study results showed that the costoclavicular brachial plexus block by the medial approach is not better than the lateral approach in terms of performance time and number of needle manipulations and is difficult to perform. However, the block success rate and block onset time were similar, and the medial approach is not inferior to the lateral approach to achieve the desired block. We also assessed the diaphragm functions and found that none of the patients had phrenic nerve palsy. We further found that obesity and increased plexus depth add to the difficulty and increase the time required to perform the block. In the absence of any clear advantage of the medial approach over the conventional approach, we cannot recommend the medial approach over the lateral approach.