Introduction
Methotrexate (MTX) is a chemotherapeutic agent from the antimetabolite group. It is used in hematological malignancies such as leukemia, lymphoma, and many types of cancer such as osteosarcoma, head tumors, breast cancer, neck tumors, and also in inflammatory and rheumatic diseases [1]. MTX affects the cell cycle and cells stop in the S phase [2]. MTX inhibits the enzyme dihydrofolate reductase. Inhibition of tetrahydrofolate synthesis decreases the biosynthesis of thymidylate and purine nucleotides. This inhibition blocks DNA and RNA synthesis [3–6]. MTX treatment may cause various side effects. One of the major side effects is hepatotoxicity [7, 8]. The mechanism of liver damage related to MTX treatment is unknown; however, there are some hypotheses. MTX inhibits nicotinamide adenine dinucleotide phosphate (NADPH)-dependent mitochondrial enzymes. These mitochondrial enzymes are the main enzymes that produce NADPH. MTX decreases NADPH level, which is essential for the enzyme glutathione reductase. Glutathione reductase maintains the reduced glutathione level, which is an important protectant against reactive oxygen species. After MTX use, NADP levels and glutathione levels decrease; this event sensitizes hepatocytes to reactive oxygen radicals and causes hepatocyte damage [1]. MTX is mostly metabolized in the liver and excreted through the kidney. 7-hydroxymethotrexate is the main metabolite of MTX. Within 24 hours of ingestion, 7-OH methotrexate is excreted by renal filtration and tubular reabsorption. Various amounts can be eliminated through the bile [9, 10].
Vitamin E has 8 derivatives, which are 4 tocopherols and 4 tocotrienols [11–13]. α-tocopherol is the strongest derivative of vitamin E activity [14]. Antioxidant activity is the essential and very important function of vitamin E. The cell membrane phospholipids have unsaturated fatty acids. They can be oxidized spontaneously, or after the challenge reaction of oxidant molecules they can be converted into peroxide derivatives. This mechanism is called lipid peroxidation [15, 16]. Vitamin E inhibits this lipid degradation reaction and stops free radicals. It protects membranes against oxidative damage and provides membrane stability [17, 18]. Because of this feature, it is known as a chain-breaking antioxidant [16, 19]. Vitamin E binds oxygen radicals before they damage the cell membranes, nucleic acids, and cell organelles. It has been revealed that in cases of vitamin E deficiency, free radicals damage cell membranes, and the flow of calcium ions into the cell increases [20, 21].
Apoptosis is a specific cellular death mechanism which is genetically programmed and controlled. When cells complete their life period or are damaged, apoptosis provides the elimination of these damaged cells [22]. This type of apoptosis occurs under physiological conditions and it is also called physiological cell death [23]. After the signal for apoptosis, many changes occur in the cell such as shrinking shape, condensation of the nucleus, cytoskeletal breakdown, damage of the nuclear membrane, and fragmentation of nuclear DNA [24]. Various changes occur during apoptosis in the outer surface of apoptotic cell membranes. The most prominent change is the translocation of phosphatidylserine units.
The use of antioxidants increases day by day to reduce the cytotoxic effects of chemotherapeutic agents. Our goal was to analyze the protective impact of vitamin E, as a strong antioxidant agent, against liver damage caused by MTX.
Material and methods
Ethics approval
This study was carried out with the approval of the Karadeniz Technical University Local Animal Ethics Committee (No. 201238). The rats were obtained from the Animal Surgical Research Center of the Medical Faculty of Karadeniz Technical University. All experimental procedures were performed in the Animal Surgical Research Center of the Medical Faculty of Karadeniz Technical University.
Animals
This study was carried out with the approval of the Karadeniz Technical University Local Animal Ethics Committee (No. 201238). The rats were obtained from the Animal Surgical Research Center of the Medical Faculty of Karadeniz Technical University. All experimental procedures were performed in the Animal Surgical Research Center of the Medical Faculty of Karadeniz Technical University.
Thirty-two male Sprague Dawley rats (8 weeks old, 200-251 g) were used. All animals were kept in the laboratory at an average of 22 ±2°C temperature and the relative humidity was 50 ±5% on average. The rats were kept in a 12 hours light, 12 hours dark cycle. The rats were given tap water with unlimited access and were fed with a standard regime. All animals were weighed daily and doses were calculated according to daily weight measurements.
Chemicals
Methotrexate was purchased from Koçak Farma (50 mg/5 ml injectable solution, Koçak Farma, Tekirdağ, Turkey). Vitamin E was manufactured by Evigen (2 ml Aksu Farma, Istanbul, Turkey).
Experimental design
The rats were randomly split into four groups, each with 8 rats. The first group (control group) received only saline 2 ml (intraperitoneally – i.p.) for 5 days. The second group (MTX group) received 20 mg/kg MTX (i.p.) only on the initial day of the study. In the third group (MTX + vitamin E group): 20 mg/kg MTX (i.p.) was applied only on the first day of the study and 100 mg/kg vitamin E (i.p.) was applied for 5 days during the study. In the fourth group (Vitamin E group) 100 mg/kg of vitamin E (i.p.) was given for five days during the study. At the end of the 5th day, exsanguination under the anesthesia method was used to sacrifice rats. Liver tissues were taken out and weighed. The right lobes of the liver were used for histopathological examinations, and the left lobes of the liver were used for flow cytometry.
Histopathological process
Liver tissue samples were fixed in 10% neutral formaldehyde. After an increasing alcohol series, liver tissues were cleared in xylene. Then all tissues were embedded in paraffin and 5 µm thickness sections were taken from all paraffin blocks with a microtome (Leica RM 2255). Two consecutive section sets were taken from each paraffin block. All sections were stained with hematoxylin-eosin (H&E) and Periodic acid Schiff (PAS). A light microscope was used to evaluate the sections (Olympus BX-51; Tokyo, Japan). All slides were examined by an independent histologist. In the H&E stained sections, five areas were selected by random sampling and the liver damage was semiquantitatively evaluated and degeneration of hepatocytes, pycnotic nucleus, dilatation of sinusoids, infiltration of mononuclear cells, were examined according to a scoring system [25, 26]. Description and semiquantitative scoring system for histological damage graded: 0 to 3: 0 – none, 1 – mild, 2 – moderate, and 3 – severe. In the PAS-stained sections, the glycogen accumulation of hepatocytes was examined.
Flow cytometry
Experimental process
Paraffin blocks were deparaffinized chemically and mechanically using the method modified from Hedley [27, 28]. Liver tissue samples were suspended and the suspension was passed through a nylon DNA mesh and particles were filtered (Spectramesh-nylon, 50-micron Backman-Coulter). The BD Pharmingen Pe ANNEXIN V Apoptosis Detection Kit was used to stain each suspension (Cat: 559763, Lot: 5306537). Flow cytometry analysis was performed with the BD Accuri C6 Cytometer device and apoptosis peak percentage rates detected in histograms were evaluated using the analysis program BD Accuri C6 Cytometer software (Version 1.0.264.21).
Annexin V method in apoptosis and apoptotic index
In apoptotic cells, phosphatidylserine is displaced from the inside to the outside of the plasma membrane. This is one of the major changes of apoptosis [29]. Annexin V is a distinctive protein that has the ability to bind to phosphatidylserine. Apoptotic cells can be described by defining them with a fluorescent compound such as FITC, which can connect to phosphatidylserine that is translocated to the cell’s outer surface. Flow cytometry can be used to determine the rate at which the FITC-Annexin-V complex binds to phosphatidylserine on the cell surface. If the rate of association of the FITC-Annexin-V complex with phosphatidylserine is increased, it means that the percentage of apoptotic cells is increased [30–32]. Annexin V has higher sensitivity and specificity to separate normal cells from apoptotic cells [33]. The apoptotic index is calculated by dividing the percentage of Annexin V-positive cells by the total number of cells in the gate [29].
Statistical analysis
SPSS Statistics (version 22) and Microsoft Excel programs were used for data analysis. The Kruskal-Wallis test was used for comparisons between groups. The statistical significance level was accepted as p < 0.05. The Bonferroni-corrected Mann-Whitney U test was used for paired comparisons in those with a significant Kruskal-Wallis test; the significance level was accepted as p < 0.0083. For histological liver damage scores, data were analyzed by one-way ANOVA and post hoc comparisons were done using Tukey’s multiple comparison test. P-value < 0.05 was accepted as significant.
Results
Liver weight measurement
According to a statistical analysis of liver weights of all groups, there was no statistically significant difference (p = 0.54) (Table 1).
Histopathological results
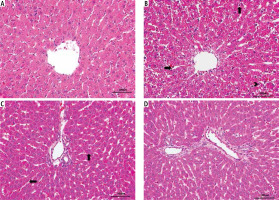
According to analyses of H&E staining, normal liver morphology was observed in the Control and Vitamin E groups. In the MTX group, atrophy in hepatocytes, sinusoidal dilatation and pycnotic nucleus were observed. Pycnotic nucleus sign was significantly higher compared to the control group. In the MTX + vitamin E group, hepatocyte cytoplasm was significantly improved. The number of pycnotic nuclei decreased. Dilatation in sinusoids improved compared to the MTX group (Fig. 1).
Fig. 1
Light microscopic sections of liver in different groups (H&E staining, 400×). A) Control group: Normal liver morphology observed in control group A. B) MTX group: (>) Atrophy in hepatocytes, (→) Sinusoidal dilatation, (↑) Pycnotic nucleus was observed. C) MTX + vitamin E group: (←) A decrease in sinusoidal dilatation, (↑) A significant improvement in the hepatocyte cytoplasm was observed. D) Vitamin E group: Normal liver morphology observed as similar to control group
According to histologic damage score analysis, degeneration of hepatocytes, pycnotic nucleus, dilatation of sinusoids, infiltration of mononuclear cells, and hemorrhage were higher in the MTX group compared to Control and Vitamin E groups. In the MTX + vitamin E group, the damage was lower compared to the MTX group (Table 2).
Table 2
Analysis of histological liver damage score for all groups
| Groups | Hepatocyte degeneration | Pycnotic nuclei | Sinusoidal dilatation | Mononuclear cell infiltration | Hemorrhage |
|---|---|---|---|---|---|
| Control | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b |
| MTX | 2.88 ±0.353a | 1.00 ±0.0a | 1.88 ±0.834a | 2.88 ±0.353a | 3.00 ±0.0a |
| MTX + vitamin E | 1.50 ±0.534c | 0.12 ±0.353c | 0.12 ±0.353c | 2.00 ±0.534c | 0.25 ±0.462c |
| Vitamin E | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b | 0.0 ±0.0b |
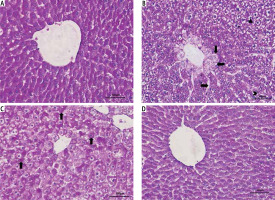
In PAS staining, normal liver morphology and glycogen accumulation were observed in the Control and Vitamin E groups. In the MTX group, glycogen accumulation in hepatocytes was decreased, pycnotic nuclei were observed in hepatocytes, and vacuole formation was observed in the hepatocyte cytoplasm. Sinusoidal dilatation and hydropic degeneration (vacuolization/cellular swelling) in hepatocytes around the vena centralis were observed. In the MTX + vitamin E group there was a significant improvement in hepatocyte cytoplasm compared to the MTX group and an increase in glycogen accumulation in hepatocytes. Sinusoidal dilatation was decreased (Fig. 2).
Fig. 2
Light microscopic sections of liver in different groups (PAS staining, 400×). A) Control group: Normal liver morphology and glycogen accumulation observed in control group A. B) MTX group: (Δ) Pycnotic nucleus, (←) Vacuole in the hepatocyte cytoplasm, (→) Sinusoidal dilatation, (>) Glycogen accumulation in hepatocytes decreased, (↓) hydropic degeneration in hepatocytes around the vena centralis was observed. C) MTX + vitamin E group: (↑) There was a significant improvement in hepatocyte cytoplasm compared to the MTX group and an increase in glycogen accumulation in hepatocytes. D) Vitamin E group: Normal liver morphology and glycogen accumulation observed as similar to control group
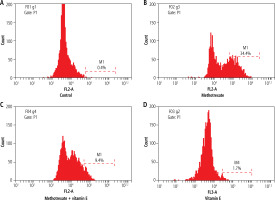
Apoptosis
According to apoptotic index results, the apoptotic index percentage (AIP) was 0.4% in the control group. In the MTX group, the toxic effect of MTX was significant and AIP was increased to 34.4%. In the MTX + vitamin E group, AIP decreased and it was 9.4%. In the Vitamin E group, AIP was 1.7%, similar to the control group.
According to a statistical analysis of all groups, there was a statistically significant difference between all groups (p < 0.05). The AIP in the MTX group was significantly higher compared to the other groups. The AIP in the MTX + vitamin E group was significantly lower compared to the MTX group (p < 0.05) (Fig. 3).
Discussion
Methotrexate has been used for many years in cancer chemotherapy, inflammatory, rheumatological, and autoimmune diseases [1]. Hepatotoxicity is a major side effect of MTX treatment. Hepatotoxicity is related to the dose taken. It is frequently observed that high-dose MTX causes hepatotoxicity, so cancer treatment with MTX is negatively affected [34].
The mechanism of hepatotoxicity that occurs after MTX treatment has not been fully defined [1]. Previous studies in the literature have observed that oxidative stress is a reason for MTX-induced hepatotoxicity, MTX-induced nephrotoxicity, and MTX-induced GIS toxicity [1, 35, 36]. MTX decreases the reduced glutathione levels, which is an essential antioxidant that protects cells against reactive oxygen radicals [1]. Reactive oxygen products are continuously synthesized and destroyed in the liver. Oxidative stress, released reactive oxygen products and various cytokines cause damage to Kupffer and Ito cells around hepatocytes. If this effect continues, the hepatocytes go to apoptosis, and finally, fibrosis occurs [37, 38]. While chronic exposure to low-dose MTX causes fibrosis and cirrhosis, high-dose MTX causes sudden deterioration in liver function tests [39]. Various studies have shown that MTX treatment in the liver increases apoptosis and they used different antioxidants to improve this apoptosis and hepatotoxicity. Bu et al. [40] observed an increase of apoptosis in the liver with MTX and investigated that using rhein as an antioxidant that decreased apoptosis. Mahmoud et al. [41] studied MTX and found that apoptosis increased in the liver. They administered berberine as an antioxidant to reduce apoptosis in the liver. Also, Ali et al. [42] used MTX and found that apoptosis increased in liver hepatocytes. They administered chrysin as an antioxidant and reported that the apoptosis decreased with use of an antioxidant. Ali et al. [8] used MTX in the liver and observed that apoptosis increased in hepatocytes. They administered chlorogenic acid as an antioxidant and found that the apoptosis decreased with use of an antioxidant. In another study, Mukherjee et al. [43] used MTX in the liver and observed that apoptosis increased. They administered pomegranate as an antioxidant and found that the apoptosis decreased. In a different study, Türk et al. [44] used MTX and observed that apoptosis increased in the liver. They administered zingerone as an antioxidant and reported that the apoptosis was decreased by using an antioxidant. In our study, we evaluated the response of vitamin E against the hepatotoxic effect of MTX.
Vitamin E has been known for its strong antioxidant effect for a long time and this effect has been evaluated in various treatment modalities [20, 21, 45]. In numerous studies, vitamin E has been used for preventing different damage that may occur in the liver and other tissues [20, 21, 46-50].
Vitamin E shows antioxidant activity by neutralizing free radicals and oxygen radicals [17]. In ethanol-dependent liver damage, free radicals have toxic effects on the liver and vitamin E protects the liver from these effects. In alcoholic liver disease, vitamin E levels decrease. Vitamin E treatment against the effects of alcohol has provided clinical benefits [21, 51, 52]. If the damage of the liver is not identified in the early stages, irreversible damage begins from hepatocyte degeneration and the liver goes to fibrosis and cirrhosis [53]. In cirrhosis, vitamin E levels have been found to be significantly low, and it is thought that vitamin E deficiency may play a role in cirrhosis etiopathogenesis [54, 55].
As a strong antioxidant, various studies have shown that vitamin E decreases apoptosis in the liver and other tissues. Abdel-Daim et al. [47] demonstrated that fipronil induced hepatotoxicity and reported that apoptosis increased in hepatocytes. They used vitamin E to reduce apoptosis in the liver. Ibrahim et al. [56] administered vitamin E for tramadol-induced hepatotoxicity. They observed a decrease in apoptosis in hepatocytes. Shen et al. [57] examined CCL4-induced hepatotoxicity and the increase of apoptosis in liver hepatic stellate cells. They found that vitamin E decreased apoptosis in stellate cells of the liver. Zhang et al. [58] investigated phoxim-induced hepatotoxicity and observed that apoptosis increased in hepatocytes. They reported that vitamin E decreased apoptosis in hepatocytes. Cuce et al. [59] demonstrated cyclophosphamide-induced hepatotoxicity and found that apoptosis was increased in hepatocytes. They administered vitamin E for its antioxidant effects and observed that apoptosis decreased in hepatocytes.
In the literature, MTX has been given to rats in different doses and ways of application. In our study, we administered MTX in a single dose of 20 mg/kg i.p. [1, 60]. In one study MTX 20 mg/kg i.p. was administered as a single dose, then L-carnitine 500 mg/kg was used for five days, and liver tissues were examined histologically. Degeneration of hepatocytes, congestion of vascular structures, and dilatation of different sinusoids were observed in the MTX group [60]. Similar to the results of this study, we found hepatocyte degeneration, sinusoidal dilatation, and pycnotic nucleus findings in the MTX group. Pınar et al. [25] observed that necrosis in hepatocytes was increased, inflammation was increased, and vascular congestion was increased in the MTX group, and it was found that the antioxidant tempol prevented these effects. In our study, we used vitamin E, an antioxidant, against the hepatotoxicity of MTX. Similar to the results in this study, we observed that the use of vitamin E reduced liver damage. Tunali-Akbay et al. [61] reported that in the MTX group, pathological findings – degenerated hepatocytes and inflammatory cell infiltration – were observed in the microscopy, and histopathological improvement was achieved in the liver tissue by giving resveratrol with MTX. Dalaklioglu et al. [62] reported that in the MTX group degeneration of hepatocytes, various vascular congestion, and dilatation of sinusoids were seen in the liver. They used resveratrol as an antioxidant and observed that the tissue damage induced by MTX was improved. These findings were similar to our study. Akbulut et al. [63] reported that glycogen accumulation was lower in the MTX group compared to the control group. They used 3 different antioxidants; one of them was amifostine, the second was ascorbic acid, and the third was N-acetylcysteine, and they found that the glycogen accumulation in the liver was improved by their antioxidant effects. Again in a different study, Kose et al. [64] noted that the microscopic damage score was significantly increased with MTX treatment in rat liver, but the score was decreased with montelukast treatment. In the PAS stain, the glycogen accumulation in the MTX group was decreased. In our study, we were able to reduce the damage of MTX with vitamin E treatment and our PAS stain results were similar to this study. In our study, we also used flow cytometry and Annexin V for investigating the change of apoptosis in different groups. Chen et al. [65] studied Annexin V and flow cytometry in an alcohol-induced hepatotoxicity experiment. They used hepatocyte cell culture and used S-allyl-l-cysteine as an antioxidant. They reported that alcohol increased the apoptosis in hepatocytes and by using an antioxidant the apoptosis decreased. They used flow cytometry to investigate the change in apoptosis value. Our apoptotic index results were similar to this study. Liu et al. [66] studied Annexin V and flow cytometry in an ischemia-reperfusion model of rat liver. They used ascorbic acid (AA) and investigated the antioxidant effect of AA with flow cytometry and histologically. They determined that using AA reduces the apoptosis of hepatocytes. Our findings in flow cytometry were similar to this study.
In conclusion, MTX is a hepatotoxic drug. Hepatotoxicity in cancer chemotherapy or the treatment of other diseases causes discontinuation in chemotherapy and prolongation of the treatment period. Long-term treatment can be dangerous or fatal for patients [67]. Based on our histopathological and flow cytometry results, hepatoxicity that occurs with MTX can be ameliorated with vitamin E. The combination of MTX with vitamin E will be helpful for the maintenance of treatment. Further studies can be designed by using different doses and durations.