Summary
Atherectomy was found to facilitate endovascular intervention in this retrospective review of 405 patients (447 limbs) with Rutherford class 4–6 chronic limb-threatening ischemia with freedom from major amputation of 94.5%, target lesion revascularization of 55%, and surgical conversion of 2% at 24 months.
Introduction
In 2003 the US Food and Drug Administration approved the first atherectomy device (SilverHawk Plaque Excision System; Medtronic, Minneapolis, MN) for use in the treatment of peripheral arterial disease (PAD) [1]. The device was designed to be used as an adjunct to reduce plaque burden and facilitate subsequent endovascular intervention. The theory behind atherectomy is that plaque removal prior to endovascular intervention will improve long term patency, reduce the incidence of dissection and potentially reduce stent usage. Multiple atherectomy devices are now available, and atherectomy has been increasingly used as an adjunct to endovascular intervention [2, 3]. The use of atherectomy in peripheral vascular interventions in the United States has increased significantly in recent years [4–6]. The factors driving this increase and the final role for atherectomy remain unclear [4]. There are some publications showing that atherectomy may be associated with lower rates of bail-out stenting [7, 8]. Other authors feel that the role of atherectomy is unproven and that the increased use of the technology is driven primarily by high reimbursement for the procedure [9]. There is also concern that atherectomy, along with other endovascular interventions, may be associated with inferior results compared to open surgery due to embolization and loss of distal runoff from the intervention itself or subsequent occlusion [10–12]. Many of the reports on atherectomy include patients with both claudication and chronic limb-threatening ischemia (CLTI). In our practice, we find atherectomy particularly useful, and least controversial in patients with CLTI. These patients have more extensive multilevel disease, often with long segment occlusions and tibial involvement. We believe that atherectomy is particularly useful for recanalization of long arterial occlusions. It increases the ability to recanalize popliteal and tibial vessels with minimal stent use and facilitates angiosome-directed revascularization. We feel that it allows us to extend endovascular reconstruction to a broader range of patients and anatomical configurations. Open surgery in patients with CLTI more often involves femoral-tibial bypass, with its attendant need for a long segment of good quality autogenous vein and increased risk of wound complications and postoperative immobility. Therefore, we prefer atherectomy as a first approach with endovascular intervention in elderly frail patients or those without an adequate saphenous vein.
Material and methods
Subjects, data collection and endpoints
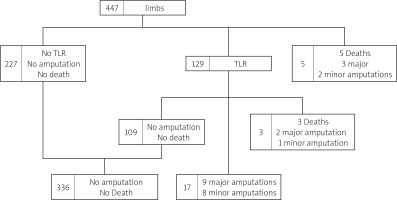
We performed a retrospective analysis of 432 consecutive patients (474 lower limbs) with CLTI who underwent atherectomy along with endovascular revascularization of the infrainguinal arteries. Patients treated in either an office-based laboratory (OBL) or hospital (H) setting between December 1, 2019 and the end of December 31, 2021 were included to allow for adequate follow-up. They were retrospectively entered into a vascular database. Periprocedural and follow-up data were retrieved from hospital/OBL electronic medical records. All procedures were performed or supervised by vascular surgeons in a high-volume vascular center. The long-term follow-up was based on ambulatory check-ups. Since deidentified data were analyzed, no ethics committee permission or patients’ consent was required. Primary endpoints were target lesion revascularization (TLR), total and major amputations and death. Minor endpoints were relief of rest pain and healing of wounds. TLR was defined as revascularization of the segment of the artery which was previously treated using an atherectomy device. Amputation was regarded as major when performed at the level of the ankle joint or higher.
Procedural characteristics and pharmacological regimen
Procedures were performed using a laser (Excimer, Phillips), orbital (Diamondback 360, CSI), rotational (Rotarex, BD) or directional (Hawk 1, Medtronic) atherectomy device. The choice of atherectomy device was based on the surgeon’s preference. Generally, rotational atherectomy was preferred in the case of highly calcified lesions and laser atherectomy was chosen in non-calcified lesions where the plaque was “soft” and atheromatous and in cases of single-vessel tibial run-off to minimize the risk of peripheral embolization. Atherectomy was used to prepare the vessel for endovascular intervention and in all cases was followed by angioplasty with or without stenting. Post-procedurally, all the patients received dual antiplatelet therapy (aspirin 81 mg/day with clopidogrel 75 mg/day) and statin therapy.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR). According to the distribution assessed with the Shapiro-Wilk test, normally distributed continuous data were compared using the two-sample t-test and those not normally distributed using the Mann-Whitney U-test. Categorical variables are presented as counts with percentage and compared using the χ2 test. Amputations and TLR are presented using Kaplan-Meier estimates. The log-rank test was used for comparison between Kaplan-Meier curves. Estimated 1- and 2-year TLR-free probability is presented with standard error (SE).
To discern differences in amputation-free survival time (the outcome variable), a stepwise forward variable selection algorithm employing the greedy Wilks method was used, following the methodology outlined by Mardia et al. [13]. The Rutherford classification, comprising classes 4, 5, and 6, was selected as the independent variable. This selection was based on the variable’s ability to minimize Wilks’ lambda, ensuring its statistical significance. Subsequently, patient age was incorporated as a covariate, also based on its ability to further reduce Wilks’ lambda.
A p-value < 0.05 was regarded as significant. Statistical analysis was performed with IBM SPSS v. 28 (IBM Corp.).
Results
Clinical characteristics
During the study period 432 patients (474 limbs) underwent atherectomy prior to angioplasty or stenting. Periprocedural success was achieved in all cases; no distal embolization or dissection related to atherectomy use was observed. Twenty-seven (6.25%) patients had no follow-up data available and were excluded from further analysis. This resulted in 405 patients and 447 limbs available for analysis. The baseline patient characteristics are presented in Table I and show the typical profile of the PAD population. Of the 447 limbs treated, 123 (27.5%) were Rutherford 4, 284 (63.5%) were Rutherford 5, and 40 (9%) were Rutherford 6.
Table I
Demographic characteristics
Anatomic characteristics
Overall, 1190 lesions were treated in 447 limbs with an average of 2.66 ±1.02 lesions treated per limb. Fifty-six percent of lesions (56.3%) were in the femoral-popliteal distribution and 43.7% were infrapopliteal. Two hundred eighty-two limbs (282, 63.1%) had lesions treated in both the femoral-popliteal and tibial distribution. Only infrapopliteal lesions were treated in 67 (15%) limbs. Six hundred eighty-one lesions (681, 57.2%) were total occlusions. There were 198 (29.1%) occlusions < 10 cm in length with 191/198 being localized to the infrapopliteal segment, 109 (16.0%) occlusions 10–20 cm and 374 (54.9%) over 20 cm in length.
Procedural details
Laser atherectomy was performed in 71.7% (853), orbital in 27.5% (327), directional and rotational each in less than 1% of lesions (6 and 4, respectively). Stents were placed in 25% (91) of femoral popliteal interventions and < 1% (3) of tibial interventions. One hundred ninety-three (193 patients) (224 legs) were treated in the office-based laboratory, whereas 212 patients (223 legs) were treated in the hospital. Characteristics of the patients treated in each facility are presented in Table I. Patients treated in the hospital were significantly older (p < 0.001) and more commonly had Rutherford 6 ischemia (17% vs. < 1%; p < 0.001). There were more Rutherford 5 patients treated in the OBL (72.8% vs. 54.3%; p < 0.001).
Follow-up
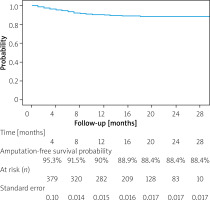
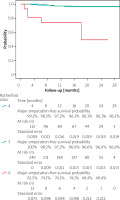
Median follow-up of the 407 patients was 16 (IQR, 8–21) months. During the observation period 20.2% (82) of patients died. Overall, 43 (10.6%) patients, representing 9.6% of limbs, required amputation, with 18 (4.4%) being above the ankle and 25 (6.2%) restricted to the foot. Median amputation time was 5 (IQR: 2–7.5) months from the initial procedure. One- and two-year estimated amputation-free survival probability was 90% (SE = 0.015) and 88.4% (SE = 0.017), respectively. Major amputation-free survival probability was 95.8% for 1 year (SE = 0.011) and 94.5% for 2 years (SE = 0.013). These data are presented in the form of a Kaplan-Maier curve in Figure 1. No significant difference in 1- and 2-year estimated amputation-free survival probability was observed between different atherectomy devices (p = 0.39 and p = 0.34, respectively).
Reinterventions
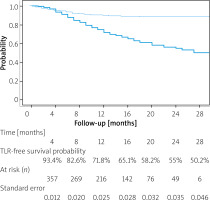
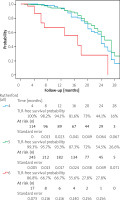
One hundred twenty-nine treated legs (28.9%%) underwent TLR with the mean re-intervention time being 9.3 ±5.4 months. One- and two-year TLR-free survival probability was 71.8% (SE = 0.0025) and 55% (0.035), respectively. The data are summarized in the form of a Kaplan-Maier curve in Figure 2. Claudication without CLTI was the indication in 43 (9.6% limbs, while 86 (19.2%) limbs underwent re-intervention for recurrent CLTI. This included 6 cases of persistent gangrene (1.5%) and 4 (0.9%) newly acquired gangrenous lesions. Reintervention to heal persistent (40) or newly acquired (21) wounds occurred in 61 (13.6%) limbs. Seventeen (3.8%) limbs underwent another procedure to treat persistent (2) or recurrent (15) rest pain. Overall, 6 reinterventions were endovascular and 11 were open surgical procedures: 10 by-passes and 1 open endarterectomy. Most open reinterventions were done in Rutherford 4 patients (9), with 1 surgery in Rutherford 5 and 1 in a Rutherford 6 patient. Distribution of interventions performed on CLTI limbs is summarized in Figure 3. No significant difference in 1- and 2-year estimated TLR-free survival probability was observed between different atherectomy devices (p = 0.28 and p = 32, respectively).
Results by Rutherford class
121/123 (98.3%) Rutherford 4 patients were relieved of their rest pain by the initial intervention. In follow-up, 6 (4.9%) patients required amputation and 46 (37.4%) required reinterventions but avoided amputation.
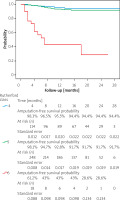
Among Rutherford 5 legs, 181 (63.7%) wounds healed and 78 (27.5%) were significantly improved during follow up, while 8.8% were unimproved or required amputation. Results in Rutherford 6 patients were not as good, with improvement in 70% of Rutherford 6 limbs with 6 (15 %) healed completely, and 22 (55%) significantly improved. Twelve limbs (30%) did not improve or were amputated. Amputation, major amputation, as well as TLR-free survival probability by Rutherford class are presented in Figures 4–6. Total amputations, as well as major amputations, were more commonly performed in Rutherford 6 than either Rutherford 4 or 5 patients (p < 0.001 and p < 0.001, respectively). Total amputation estimated probability was higher in Rutherford 5 patients than in patients with Rutherford class 4 (p < 0.04), although the major amputation rate was no different (p = 0.38). TLR was done more often in Rutherford 4 than Rutherford 5 patients (p = 0.003) and in Rutherford 6 than 5 patients (p = 0.045). There was no significant difference in TLR rate between Rutherford 4 and 6 patients (p = 0.75).
Results by location of procedure
Comparison of patients treated in the OBL and H showed no significant difference with regard to amputation and TLR rate (p = 0.15 and p = 0.13; respectively). However, there was a significantly higher mortality rate among patients treated in the H (p < 0.001).
Discriminant analysis of Rutherford class and patient age in predicting amputation-free survival time
In an analysis examining factors affecting amputation-free survival among patients with CLTI, the Rutherford classification, particularly the Rutherford 6 class, emerged as a significant predictor. This category demonstrated a notable decrease in Wilks’ lambda to 𝜆 = 0.81, with an F = 3.15 and a highly significant p-value (p < 0.001), indicating a strong association between Rutherford 6 class and reduced amputation-free survival times compared to Rutherford 4 and 5. While the addition of patient age into the model did reduce Wilks’ λ further to 0.75 and was statistically significant (p = 0.018), the marginal improvement with an F = 1.25 and p = 0.177 suggested that age did not significantly enhance the model’s predictive power beyond the Rutherford classification.
These findings highlight the predominant influence of Rutherford 6 class in predicting shorter amputation-free survival. While age remains an important consideration, its impact is secondary to the severity of ischemia in terms of clinical decision-making for these patients.
Discussion
This single center experience documents good initial and mid-term success using atherectomy as an adjunct to endovascular intervention in a large group of patients with advanced lower extremity ischemia. The procedures were performed on an elderly patient population with extensive, multilevel atherosclerotic disease and CLTI. The 20% 1-year mortality highlights the importance of immediate and midterm success as opposed to a focus on late patency and durability in the population. We observed good mid-term limb salvage rates and 2-year TLR-free survival probability (55%). While one might argue that the TLR rate was high, we believe the fact that the majority of reinterventions were endovascular with only 11 open procedures required was an advantage to this approach. To the best of our knowledge, our study presents the follow-up for the largest group in which atherectomy was restricted to patients with CLTI.
In our practice, atherectomy is used to “prepare” the target vessel optimally for subsequent endovascular intervention. This includes facilitating angiosome-directed revascularization by opening total tibial occlusions, reducing the total burden of atherosclerotic disease prior to angioplasty, reducing the frequency of dissection and stent placement particularly in the popliteal and tibial vessels, and potentially improving the long-term results of endovascular intervention. While we do not have data addressing this final point, our data support the efficacy of atherectomy for the other objectives.
The importance of angiosome-directed revascularization in patients with Rutherford 5 and 6 disease is generally accepted [14, 15]. This is not always possible with open surgical revascularization. We believe that the use of atherectomy to recanalize occluded tibial vessels, often over areas of > 10 or even > 20 cm, has increased our ability to accomplish angiosome-directed revascularization using endovascular techniques. Our results in Rutherford 5 patients with low amputation rates and wound healing/improvement in 90% of cases support this contention. The fact that lower, but still substantial, rates of wound healing/improvement are seen in Rutherford 6 patients may reflect differences in baseline characteristics of the two groups, both in terms of age and wound status. While our results in Rutherford 6 patients are not ideal, we did see improvement or healing in 70% of this challenging group. We feel these results justify our approach in these patients, although direct comparison with open surgical revascularization was not undertaken.
The second reason to use atherectomy is to reduce the incidence of stent use, especially below the knee. It is generally accepted that stents should be avoided in the popliteal and tibial vessels whenever possible [12, 16, 17]. Our results indicate that we have achieved this goal with only 30% of superficial femoral lesions and 1% of all infrapopliteal lesions requiring stent placement.
It seems intuitive that interventions on an atherosclerotic lesion that has been debulked or converted from a total occlusion to a stenosis would be associated with improved patency rates. While this remains our belief, our data cannot address this issue, since we do not have a non-atherectomy control group. However, we believe that our TLR rates are acceptable and as good if not better than similar publications of endovascular intervention without atherectomy for CLTI [18, 19]. The specific question of the role of atherectomy in the treatment of in-stent stenosis is the subject of an ongoing investigation by our group.
High rates of distal embolization after atherectomy and increased subsequent limb loss have been suggested by others [20–22]. However, this series establishes the fact that atherectomy can be performed safely without fear of this complication. We believe our policy of tailoring the atherectomy technique to the anatomy has contributed to our success.
There are few up-to-date studies describing selectively the results of atherectomy in CLTI patients. Most available articles describe atherectomy in populations that include a high percentage of claudicants [23]. A retrospective study carried out by Mallios et al. described the use of laser atherectomy in PVI, which was also the most commonly chosen type of device in our group [24]. The study reported a 2-year major amputation-free survival rate of 90%. However, it included both claudicants (34%) and patients with chronic limb-threatening ischemia (66%).
The LA-DEFINITIVE trial prospectively described the use of atherectomy with a drug-coated balloon in patients with claudication or chronic limb-threatening ischemia undergoing revascularization using the SilverHawk/TurboHawk (Medtronic) [25]. At 1 year, the freedom-from-major-amputation rate among CLTI patients was 93.8%, which is very similar to ours (95.8%).
The role of atherectomy, or any intervention, in patients with claudication remains a topic of controversy. A major theme in the recent professional and lay literature has been the lack of proven efficacy for atherectomy, with the implication that its use is driven by financial incentives. This narrative suggest that devices are used differently in OBL and hospital settings based primarily on reimbursement. However, to the best of our knowledge, no direct comparison of atherectomy results between H and OBL has been published. Our practice patterns and results are similar in both OBL and hospital settings, with the exception of an increased incidence of Rutherford 6 patients in the hospital setting, consistent with the severity of their wounds. As for the putative financial incentives, we would point out that any added revenue from the use of an atherectomy device is more than compensated by the savings from avoiding stent use in 75% of femoral popliteal and 99% of tibial interventions. We welcome further data critically evaluating the efficacy and economic impact or using atherectomy as an adjunct to endovascular interventions.
Our study has several important limitations. Most importantly, it has a retrospective character and single-center design. Another serious limitation is the presence of de novo, restenotic and in-stent restenotic lesions, all Rutherford class 4-6, as well as different atherectomy devices used in one group. However, we wanted to conduct an analysis of real-world experience including the whole spectrum of CLTI patients and endovascular alternatives, reflecting our practice. We are also aware that the use of only Rutherford classification is a potential drawback. Owing to the retrospective character of the study, accurate information to allow for correct WiFi and GLASS scoring was lacking [26]. Thus, the impact of possible additional factors such as run-off score and clinical staging including the ankle brachial index was not analyzed in the studied group and would require an additional prospective study.
Conclusions
We believe this is the largest series of consecutive CLTI patients treated with adjunctive atherectomy prior to endovascular intervention. Our results demonstrate acceptable mid-term results in a challenging group of patients with low amputation rates and good relief of symptoms despite the need for close follow-up and TLR rates of 45%. We believe our results confirm our bias regarding the benefits of atherectomy in this patient group and agree that further objective data on the efficacy and economic role of atherectomy in endovascular treatment of CLTI are needed.