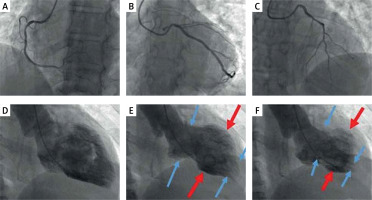
A 62-year-old woman, current smoker, without other cardiovascular risk factors, presented in a satellite district hospital with typical chest pain lasting 2–3 h and vomiting. The electrocardiography (ECG) at presentation showed ST segment elevation in precordial leads V1-V3; therefore, the patient was thrombolysed with tenecteplase. Post-thrombolysis, the patient was haemodynamically and clinically stable on dual antiplatelet therapy (aspirin and clopidogrel). A bedside transthoracic echocardiogram showed moderate left ventricular systolic dysfunction (LVEF: 40%) and that cardiac troponin T was elevated to 1000 pg/ml (normal value < 14 pg/ml). The patient was subsequently transferred to our centre for a coronary angiogram ± percutaneous coronary intervention (PCI). The coronary angiogram performed via the left transradial approach and showed unobstructed coronary arteries. Left anterior descending and left circumflex arteries had separate ostia (Figures 1 A–C). Left ventriculography demonstrated moderate left ventricular systolic dysfunction with akinesia of the midventricular myocardial segments and preserved systolic function of the basal and apical segments, an angiographic appearance compatible with midventricular Takotsubo syndrome (Figures 1 D–F). Taking a thorough patient history in the cath lab, we found that the symptoms had started after a major dispute with a first-degree family member, which perfectly fitted our diagnosis. The patient was discharged 2 days later on a β-blocker and an angiotensine convertase enzyme (ACE) inhibitor and was scheduled for cardiac magnetic resonance (CMR) imaging at 1 month post discharge. The CMR showed complete recovery of left ventricular systolic function.
Figure 1
A – Invasive coronary angiography (ICA), LAO view with unobstructed right coronary artery. B – ICA, LAO caudal view with unobstructed left circumflex artery. C – ICA, LAO cranial view with unobstructed left anterior descending artery. D–F – Left ventriculography (RAO view), in diastole (D), mid-systole (E), and end-systole (F), demonstrating akinesia of midventricular segments (red arrows) and normal systolic function of basal and apical segments (blue arrows). The left ventricle in systole resembles the ancient Greek vessel known as ‘amphora’ (wide main body and narrow base and apex) contrary to the apical ballooning subtype where left ventricle resembles the Japanese octopus trap called Takotsubo
Takotsubo syndrome (TTS), formerly known as Takotsubo cardiomyopathy, stress-induced cardiomyopathy, broken heart syndrome, and transient apical ballooning, was first described in Japan in 1990 and refers to an acute reversible heart failure syndrome that is characterized by transient LV regional wall motion abnormalities usually extending beyond a single epicardial vascular distribution, in the absence of obstructive coronary artery disease or plaque rapture in coronary angiography [1, 2]. TTS patients present with chest pain, ECG changes (ST-segment elevation, ST depression, or T-wave inversion) and troponin elevation. Overall, 1% to 2% of patients with suspected acute coronary syndrome (ACS) and 5% to 6% of all women presenting with suspected STEMI who undergo urgent angiography, are eventually diagnosed with TTS [2]. The pathophysiological mechanism of the syndrome has not been completely elucidated; however, intense sympathetic activation and elevated circulating catecholamine levels play a central role in its pathophysiology [1]. There are many potential triggers of Takotsubo syndrome, including emotional events (primary TTS) or physical and acute medical or surgical conditions (secondary TTS) [1]. Although TTS generally occurs in older adults and predominantly in post-menopausal women, cases of TTS in children and teenagers have also been described [3].
Despite the fact TTS was previously considered to be a benign condition due to the transient nature of LV dysfunction, both hospital complications in the acute phase (such as ventricular arrhythmias, acute heart failure, and cardiogenic shock) and long-term mortality are comparable to those of ACS patients [1, 2].
The 4 main anatomical subtypes of TTS are as follows: apical ballooning (75–80% of cases), midventricular (10–20%), as in our case, basal or inverted (5%), and focal (< 1%); however, other very rare variants (e.g. biventricular or isolated right ) have also been described [2, 4]. The midventricular subtype is associated with more significant cardiac output reduction and cardiogenic shock, whereas patients with the basal or inverted subtype (akinesis of basal LV segments) demonstrate less severe haemodynamic compromise [5]. In 15–25% of apical ballooning patients, on the other hand, basal segment hyperkinesis can result in LV outflow obstruction and systolic anterior motion (SAM) – induced mitral regurgitation, which can lead to pulmonary oedema and cardiogenic shock [5]. Finally, focal subtypes are more benign and normally do not cause haemodynamic complications.
Complete LV systolic function recovery, which is an essential part of the diagnosis of TTS, can occur within a few days to several weeks [1, 2]. In the absence of evidence-based treatment the optimal management relies on clinical experience and expert consensus. ACE inhibitors post-discharge are associated with a lower prevalence of TTS recurrence [6].
In conclusion, TTS represents a heterogeneous condition with regards to clinical presentation, anatomical features, and clinical course, which should always be included in the differential diagnosis of patients presenting with ACS and unobstructed coronary arteries.








