Introduction
Minimally invasive surgery has become common practice in thoracic surgery over the last decades and provides optimal results for the treatment of many thoracic diseases. However, there are still cases in which a minimally invasive surgery needs to be converted to conventional thoracotomy due to complications or anatomical reasons. The conversion from a minimally invasive operation to an open operation, especially through an emergency thoracotomy, is associated with severe postoperative pain. Closure of the thoracotomy incision is a critical step to prevent postoperative complications, such as bleeding, infection, and dehiscence. The traditional suture technique used for thoracotomy closure involves tying sutures through the intercostal muscles, which can be traumatic to the intercostal neurovascular bundle and lead to postoperative pain. In most cases, the pain intensity is moderate. However, severe pain is described by many patients. In addition, chronic pain can severely disable patients in everyday activities [1]. Chronic pain symptomatology is a common complication after thoracic operations. However, the occurrence of the post-thoracotomy pain syndrome (PPS) varies. PPS is encountered in 33–91% of cases [2–5]. To date, PPS remains one of the most common preoccupations that thoracic surgeons have to deal with in everyday clinical practice.
The aim of this report is to describe a modified, easy, and fast thoracotomy closure technique that is atraumatic to the intercostal neurovascular bundle, to avoid nerve entrapment at the time of chest closure, and suitable for cases of conversion to thoracotomy after a minimally invasive attempt.
Chronic post-thoracotomy pain and conventional thoracotomy closure techniques
Several studies have attempted to explore the common problem of PPS; however, it remains poorly understood [5–10]. There have been many reports in the literature that refer to the treatment of chronic post-thoracotomy pain, but very few of them deal with its aetiology [11]. Chronic post-thoracotomy pain is considered as a complex multifactorial condition [12]. Several factors and operative actions have been referred to as causes contributing to the PPS, such as the patient’s position during surgery, transecting muscles, crush injuries or muscle ischaemia, spreading the ribs, rib fractures, placement of a chest tube, neurovascular compression with suture during closure, and local infection/pleurisy or costochondritis/costochondral dislocation. Many other causes may also affect the pain including anaesthesia, analgesic treatment, and even patient-related factors such as age, sex, complications, etc. [13–16].
Analgesia is always required, and intrapleural and intercostal anaesthetics and systemic opioids are routinely administered. The administration of intrathoracic intraneural injection of the intercostal nerve has been abandoned since it was reported to cause hypotension, total spinal block, or a combination of both [17]. Patients undergoing thoracotomy may suffer from severe postoperative pain if analgesia is not managed appropriately. The efficacy of anaesthetic agents varies, and it remains a matter of debate [15, 18]. Moreover, thoracotomy deteriorates respiratory and pulmonary function, and this is further worsened due to postoperative pain increasing the risk of pulmonary complications [19]. Thus, adequate analgesia and regular physical therapy are always necessary [20].
It remains unclear which of the factors described above has the major part in the syndrome onset, although it is well known that pressure on nerves can cause severe and long-lasting pain. Previous literature reports have indicated that intercostal nerve damage during thoracotomy may be responsible for chronic post-thoracotomy pain [21]. The exact mechanism of nerve injury has not been clearly explained. The intercostal nerve could possibly be injured in any part of the operation – during incision, rib spreading, or thoracotomy closure. Rogers et al. performed an intraoperative neurophysiological study. They performed intercostal nerve motor-evoked potentials to identify nerve injury during thoracotomy. They demonstrated that intercostal nerve injury occurs routinely due to rib retraction during thoracotomy [12]. Muscle-sparing thoracotomy [22], in which the latissimus dorsi and serratus anterior are retracted, not divided, did not reduce the incidence of chronic pain when compared to standard posterolateral muscle-cutting thoracotomy [23]. This suggests that division of the muscle layer of the chest wall is unlikely to contribute to chronic pain.
Post-thoracotomy pain can probably be attributed mainly to the injury caused to the intercostal nerve. Conventional thoracotomy closure techniques usually include pericostal sutures, which are placed around the ribs and are highly likely to entrap and compress the underlying neurovascular bundles. Nerve entrapment causes pain and discomfort after surgery. Surgeons are always very careful not to injure the bundles because injury to the intercostal nerves is thought to be associated with long-term postoperative pain [13, 16, 24, 25].
Surgical techniques to reduce impairment of the neurovascular bundle
Several surgical techniques have been reported in the literature, aiming to reduce postoperative pain by preventing injury to intercostal nerves. Certfolio et al. tried to avoid entrapment of the neurovascular bundle during thoracotomy closure by using intracostal sutures instead of conventional pericostal sutures, placed after drilling 4 small holes in the underlying rib. Intracostal sutures seemed to be less painful than pericostal sutures in the patients’ short-term and long-term follow-up after thoracotomy [13]. Similar results have been reported in animal studies in which transcostal sutures passed through similarly drilled holes appeared to be less harmful than the standard circumcostal closure technique [19]. Bayram et al. also propose the use of intercostal sutures, which include drilling holes in both ribs and partial dissection of the intercostal muscles in order to avoid nerve compression [26].
Harvesting of an intercostal muscle (ICM) flap and leaving the muscle intact under the chest retractor can offer further reduction of the pain. Pressure from the metallic thoracic retractor on the intercostal nerve during the thoracotomy increases the possibility for postoperative pain on the thoracotomy. Avoidance of retraction on the ICM and the nerve that runs in it further reduced postoperative pain, reduced the need for analgesic agents, and helped patients to return faster to baseline activity [16, 27].
Many surgeons in several institutions have used a method that involves suturing using a large blunt needle through the narrow space between the caudal side of the rib and the intercostal neurovascular bundle, preventing strangulation of the intercostal nerve and vessels on the caudal side. Despite its everyday use for several years, Sakakura et al. first reported the technique and named it ‘edge closure (EC) technique’. The intercostal neurovascular bundle can be located by slightly separating the intercostal muscle from the rib, and it can easily be preserved successfully [28].
Other efforts to avoid injuries have been described. Mason proposed using a “tonsil” artery clamp to bluntly penetrate the intercostal muscles and pleura above and below the ribs and pulling sutures through the penetration sites with the clamp [29]. However, this technique is much more complicated, more traumatic, and demands extra time, tools, and steps.
Our technique
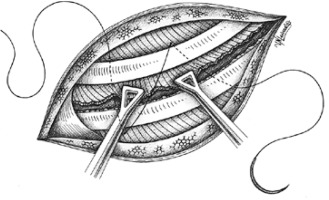
After converting a video-assisted thoracoscopic surgery (VATS) to an open one with a thoracotomy, in a way to avoid nerve entrapment at the time of chest closure, we propose a thoracotomy closure technique, which includes pericostal sutures passed between the intercostal neurovascular bundle at the caudal edge of the rib using a large blunt needle, similarly to the edge closure technique. We believe this is the best way to avoid the nerve during closure. The neurovascular bundle is safely revealed by slightly separating the intercostal muscles from the rib. The needle is inserted in the intercostal space from inside the thorax by its blunt proximal tip (where the suture is attached to the needle), in the reverse position, to successfully preserve the safety of the bundle and to avoid any possible injury to the nerve or the vessels (Figures 1, 2).
Figure 1
The neurovascular bundle is revealed by slightly separating the intercostal muscles from the rib. The needle is the passageway from inside to outside
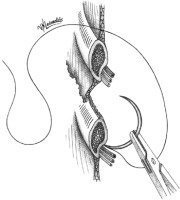
Figure 2
The needle is inserted in the intercostal space by its blunt proximal tip (where the suture is attached to the needle), in the reverse position, to successfully preserve the safety of the bundle and to avoid any possible injury to the nerve or the vessels
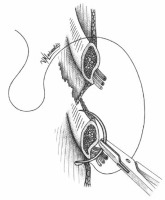
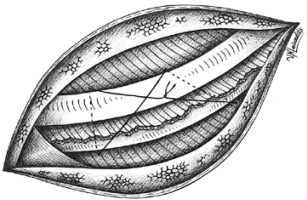
To the EC technique described from Sakakura et al. we also add a new method of placing the pericostal sutures. The main concept is to try to imitate the direction of the forces normally posed to the intercostal space by the intercostal muscles before thoracotomy. To this direction we suggest posing the pericostal sutures in a way that they form a trapezoid shape (Figures 3, 4). In this way the exterior part of the sutures has a distal part which lies vertically to the ribs and a proximal part which lies sidelong, following the direction of the exterior intercostal muscle. We believe this is the optimal way to perform a closure that best fits the normal anatomy of this region.
What is new with the modified closure technique?
Sakakura et al. used 3 or 4 sutures, and an additional Z suture was applied. Moreover, EC was used for closure of posterolateral thoracotomies as well as anteroaxillary thoracotomies. In addition, epidural anaesthesia with ropivacaine and fentanyl was performed in all patients. Conversely, in our study population only muscle-sparing anterolateral thoracotomies were performed. Furthermore, to minimize the postoperative pain no rib was cut and no intercostal muscle was harvested. None of the patients received epidural anaesthesia because a VATS was initially planned. In addition, in our case the blunt proximal tip of the needle was inserted from the intrathoracic situs with direction to the chest surface. In this way, the damage of the muscle and the pain symptomatology was lower. Furthermore, we tried to limit the number of pericostal sutures to 2 trapezoid sutures with occasionally one or 2 individual pericostal sutures, depending on the size of the thoracotomy. In this way, the pressure of the intercostal suture on the rib periosteum was further minimized.
In addition, through this technique, injuries to the surgeon’s finger can be avoided while trying to locate the needle inside the chest cavity. What is more, injury to the underlying lung is also prevented.
The modified technique in everyday clinical practice
The technique was applied to all operations that were converted from VATS to an open operation with an anterolateral thoracotomy. The technique was used in the Department of Cardiothoracic Surgery in the University of Patras since the era of open thoracotomy.
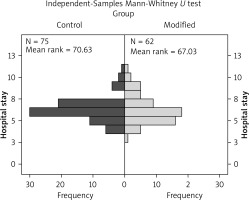
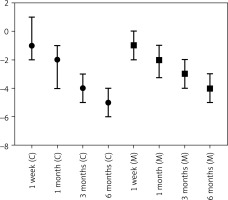
Sixty-two patients (modified group) who received the modified closure technique were recorded and compared to a historical group of 75 patients (control group) with similar baseline characteristics and tumour stadium (stage I–IIIA) who underwent a conventional thoracotomy closure technique. No significant difference (p = 0.586) was observed as far as the hospital stay was concerned (Table I, Figure 5).
Table I
The differences between the 2 groups regarding hospital stay and pain levels at different timepoints
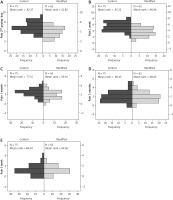
The patients receiving the modified closure technique required postoperatively less opioid medication and less rescue opioid medication. Postoperative pain was lower compared to cases in which the traditional suture technique was used, and there were no reported cases of intercostal nerve injury. The modified suture technique offered a faster and more secure closure. The classical numeric pain rating scale (0: none, 1–3: mild, 4–6: moderate, and 7–10 severe) was used to record the patients’ pain levels. On the 2nd postoperative day, the pain levels were significantly lower in the modified closure group compared to the conventional closure group. A postoperative follow-up was carried out for all these patients, and the pain scale rating was recorded at 1 week, 1 month, and 3 months after surgery (Figure 6). The recorded pain levels were significantly lower in the modified closure group until the 1st month postoperatively. After that, the described remaining pain symptoms were not significantly different. The detailed results are presented in Table I.
Method
Hospital stays as well as pain scores at various timepoints following operation were tested for normality using the Kolmogorov-Smirnov test and subsequently treated with non-parametric tests. Differences between the control (C) and modified (M) group were tested for significance using the Mann-Whitney test. The significance level (α) was set at 0.05.
Reductions of pain score were investigated by calculating the change in pain score between any time point and the second post-operative day, and expressing them as the median and interquartile range. Differences between the 2 groups were tested for significance using the Mann-Whitney test (Table II, Figure 7).
Table II
Pain score reductions from the 2nd operative day
Results
Significant differences, in favour of the modified group, were found in pain scores at the second post-operative day, at one week, and at one month following the operation. No statistical difference was found in Hospital stay between the 2 groups.
Regarding pain score reductions from the 2nd operative day, significant differences, in favour of the modified group, were found at every timepoint except for 1 month after the operation.
Discussion
Post-thoracotomy pain is an important issue that concerns thoracic surgeons in their daily clinical practice. Pain itself is multifactorial, and difficult to study and treat due to its subjective and unpredictable nature [27]. Minimally invasive surgery such as the uniportal VATS lobectomy for the treatment of early-stage lung cancer has led to less postoperative pain and shorter in-hospital stay [30]. However, even after minimally invasive thoracic operations, some patients suffer from chronic complaints, although they are less frequent and of lower intensity compared to thoracotomy [31]. Several anaesthesiological strategies to post-thoracotomy analgesia have been proposed to reduce the postoperative pain, such as thoracic epidural analgesia, intrathecal analgesia, paravertebral block, serratus anterior plane block, erector spinae block, intercostal nerve block, cryoablation, interpleural block, and interscalene block [32–35]. However, none of these techniques has offered a major significant relief of postoperative thoracic pain.
We attempted to establish a method to avoid pressure to the intercostal neurovascular bundle and consequently avoid injury to the intercostal nerve.
The proposed method effectively avoids the intercostal nerve, which remains intact and restores the anatomy of the intercostal space in a more physiological manner. The main advantages of the technique can be summed up as follows:
Notable pain reduction reported by patients who underwent thoracotomy closure according to the modified technique, compared to those who received the conventional pericostal sutures.
It is simple because it does not require extra tools to pass the suture (drills, clamps, etc.) or tools to displace the lung during suturing. It therefore offers speed and reduces surgical time of the operation, as well as postoperative complications.
The lung can be normally ventilated during thoracotomy closure, reducing the possibilities of leaving dead space in the thoracic cavity, as long as there are no air leaks from the lung.
The “trapezoid” shaped closure better ‘respects’ the physiological anatomical order, the intercostal space seals much more tightly, leaving no gaps, the lower rib is not thrusted, and the respiratory device functions much more properly.
This method also offers ease and speed to minimally invasive techniques, and in the scenario of large thoracotomy procedures, excellent apposition of the ribs is facilitated even if there are rib fractions.
Sakakura et al. reported promising results from the EC. Bleeding from injury of the intercostal vessel occurred in one case in which the technique was performed roughly. An injury of the intercostal vessels could be avoided by placing the suture as carefully as possible and by placing the blunt tip of the needle in contact with the back of the rib. This task could be made easier by exposing the caudal edge of the rib with the electrocautery. However, prolonged use of the electrocautery in the area could injure the nerve and the vessels. For the same task, Sakakura et al. suggested the use of spatulas to peel the muscle from the edge of the rib [28].
We believe that the described modified closure technique is a simple method that could reduce postoperative pain and prevent the strangulation of the intercostal nerve. An alternative to our technique could be the method that was described from Cerfolio et al., who proposed the drilling of small intercostal holes. As with our method, this technique also provides avoidance of strangulation of the intercostal nerve [13]. However, if a drill is unavailable or in cases of elderly patients with osteoporosis, our modified closure technique should be preferred [28].
However, the modified closure does not overpass the nerve injury caused by pressure posed by the rib retractor as it pulls the ribs apart. To minimize this effect, we prefer using a retractor with large branches that pull the ribs with less pressure and detach the intercostal muscle for the entire span of the rib.
Conclusion/Suggestions
In conclusion, the modified suture technique for thoracotomy closure is a simple and effective technique that is atraumatic to the intercostal neurovascular bundle. The technique offers several advantages over the traditional suture technique and has shown good clinical outcomes in our institution. It could be useful in reducing long-term postoperative pain in patients with an unplanned conversion from VATS to open thoracotomy. Further studies are needed to validate the safety and efficacy of this technique in larger patient populations.