Introduction
Similar to developments in technique and technology for percutaneous pulmonary valve implantation (PPVI), live guidance for PPVI has dynamically evolved in recent years [1, 2]. The traditional reliance on two-dimensional (2D) angiography is being replaced by rotational angiography (3DRA) and direct fusion of pre-procedure computed tomography (CT) or magnetic resonance imaging (MRI) 3D datasets [3–5]. Echocardiography (transthoracic (TTE), transesophageal (TEE) or intra-cardiac (ICE)) allows on-table assessment of a procedural outcome promising further reduction of contrast and radiation burden [6–8].
Aim
We report the integration of fusion with MRI 3D reconstruction, 3DRA, and ICE to optimize contrast and radiation reduction in PPVI.
Case report
A 45 kg 11-year-old girl with a history of repaired tetralogy of Fallot was referred to our center with severe pulmonary regurgitation. She had a surgical pulmonary valve replacement with a 25 mm Sorin Mitroflow valve in 2012 (Sorin Group USA Inc, Arvada, Colorado). Routine surveillance TTE revealed significant stenosis of the bio-prosthetic valve with a peak instantaneous gradient of 90 mm Hg. Magnetic resonance imaging showed a severely dilated right ventricle (RV) with indexed end diastolic volume of 180 ml/m<sup>2</sup> and low normal systolic function (ejection fraction of 44%). Moderate regurgitation of the pulmonary valve bio-prosthesis was visualized (25% regurgitation fraction). There was congenital interruption of the inferior caval vein with drainage via azygous continuation to the superior caval vein. The coronary arteries were not well visualized. After discussion at a multi-disciplinary meeting, the patient was accepted for PPVI.
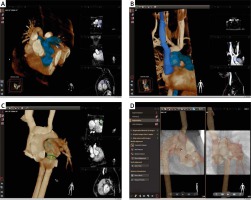
Prior to the procedure, her MRI scan was imported and manipulated on a dedicated workstation (VesselNavigator, Philips Healthcare, Best, Netherlands) to highlight the target structures (Figure 1).
Figure 1
Segmentation and fusion of three-dimensional reconstruction. Pre-catheter magnetic resonance imaging scan was manipulated to expose (in blue) target structures (A) and vessels used for registration (B). Two (blue) rings were placed to indicate origins of branch pulmonary arteries and a green ring to mark the bioprosthetic valve (C). A soft wire placed in the aorta served as reference for alignment of the three-dimensional reconstruction remonstration with fluoroscopy images in right lateral and posterior-anterior projections (D)
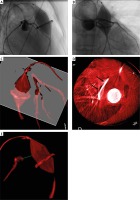
Under general anesthesia, an 8-Fr sheath was introduced into the right internal jugular vein. Stored fluoroscopic images in two projections were used for direct fusion of 2D fluoroscopy and 3D reconstruction (2D–3D registration, Figure 1 D) [4, 8]. A soft wire in the aorta together with a diagnostic catheter in the superior caval vein and the radiopaque ring of the bio-prosthesis served as references for alignment of the 3D reconstruction. A right heart hemodynamic evaluation, guided by the aligned 3D roadmap, confirmed the noninvasive imaging findings with near systemic RV pressure and a 50 mm Hg peak-to-peak gradient across the bio-prosthesis. After introduction of an Amplatz 0.035” Superstiff (Boston Scientific, Marlborough, Massachusetts) wire to the left lower pulmonary artery, a 22 mm Atlas Gold balloon (Bard Peripheral Vascular, Inc, Tempe, Arizona) was positioned across the bio-prosthetic valve. The balloon was inflated to 12 atm, and 3DRA was performed with simultaneous contrast injection in the left coronary artery (LCA) (Figure 2). After analysis of the rotational image and post-processing to generate multi-planar reformats and 3D reconstruction, we determined that there was a safe distance between the LCA and the pulmonary bio-prosthesis. We did not feel that there was enough space to break the valve ring to place a larger valve without potentially compromising the LCA [2].
Figure 2
Coronary artery compression testing. Three-dimensional rotational angiography (3DRA) was performed with simultaneous selective left coronary angiography and balloon inflation across the stenotic bioprosthesis. Right anterior oblique (A) and posterior-anterior (B) frames from rotational angiography show uncompromised coronary flow. On 3D reconstruction (C, D) a plane (white) was set to expose the minimal distance between the balloon and the left anterior descending coronary artery (black arrows). It was measured at 11 mm (E)
The decision was made to proceed with valve-in-valve implantation of a 23 mm Edwards Sapien 3 valve (Edwards Lifesciences, Irvine California). Over a 0.035” Lunderquist wire (Cook Medical, Bloomington, Indiana) a 22-Fr, 30 cm Gore DrySeal sheath (W. L. Gore & Associates, Flagstaff, AZ) was fed via the right jugular vein and across the RV and bioprosthetic valve. The valve was then crimped onto an Edwards Certitude delivery system, advanced without difficulty through the DrySeal sheath, then deployed in a satisfactory position within the bioprosthetic valve ring (Figures 3 A–C).
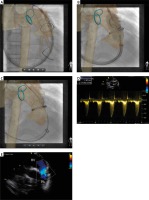
Figure 3
Percutaneous pulmonary valve-in-valve implantation and control intracardiac echocardiography. With guidance of fused three-dimensional reconstruction, the valve was smoothlydelivered (A) and implanted (B) within the bioprosthetic pulmonary valve. Various degrees ofmisalignment of the pulmonary artery branch, expressed as shift of the wire outside the blue marking ring, was observed during valve delivery (A) and implantation (B) and after removal of the stiffwire (C). However, the target zone for valve delivery remained well aligned throughout the procedure. Final evaluation with intracardiac echocardiography (D, E) showed low velocity of flow across the valve with no regurgitation
Repeat hemodynamics demonstrated only a 7 mm Hg gradient across the valve with a RV pressure of < 1/3 systemic. The delivery system was removed and the DrySeal sheath pulled back to the right atrium.
Intra-cardiac echocardiography (Acuson AcuNav, Siemens Healthcare, San Jose, California) was performed via the DrySeal sheath, showing no pulmonary regurgitation with maximum velocity of flow across the valve of 1.8 m/s (Figures 3 D, E) and no evidence of tricuspid valve damage. Hemostasis of the right internal jugular puncture was achieved immediately by two figure-of-8 sutures.
The fluoroscopy and procedural times were 28.9 min and 110 min, respectively. A total of 12 ml of contrast was injected (for the coronary assessments), which corresponds to 0.3 ml/kg. The total radiation dose expressed as air kerma and dose area product was 164 mGy and 9505 mGy·cm<sup>2</sup>, respectively.
The patient’s further course was uneventful. Control transthoracic echocardiography confirmed a satisfactory outcome of the procedure with maximum flow velocity across the Sapien valve of 2.5 m/s and trace regurgitation. The hemostatic suture was removed on the following morning and the patient was discharged home.
Discussion
Modern peri-procedural imaging techniques can be used in addition to traditional angiography to improve monitoring, guidance and evaluation of the outcomes of percutaneous treatment [4–7]. In young patients whose lifetime neoplasm risk is known to be significantly increased by the levels of radiation exposure typically required for complex procedures such as PPVI, we are duty bound to investigate the safety of minimizing radiation exposure. In addition to this, 3D guidance offers improved access and comfort for the operator, keeping the lateral cameras out of the field unless required due to an unexpected finding or complication (in particular when working from the neck), but also more predictable final results with reduction in use of contrast and radiation.
Here, we combined several imaging tools, enabling us to administer a trivial amount of contrast (12 ml in total) and a very low radiation exposure despite using 3DRA on a 12-year-old imaging platform. It is important to move away from unnecessary duplication of imaging the same structures, but rather apply a particular technique where it provides the best information. Hence, we utilized MRI for planning and live guidance, 3DRA for dynamic testing for coronary compression and finally ICE for outcome evaluation.
Non-invasive, preferably 3D imaging forms part of the routine practice to determine suitability for PPVI. The available MRI dataset was specifically processed to optimize understanding of the anatomy and plan the intervention [4, 8]. Later during the procedure, it was easily reused with the direct 2D–3D registration for live guidance, eliminating the need for diagnostic angiography and providing a roadmap for wire and catheter manipulation. A major current limitation and criticism is that it offers rigid models, which may not correspond to the dynamic conditions produced after the introduction of stiff delivery wires and systems and does not track periodic heart movement. Our case demonstrated this, highlighting the misalignment of the 3D reconstruction and the actual position of the left pulmonary artery after the introduction of the stiff delivery system (Figure 3). However, the position and orientation of the landing zone for the valve corresponded with the 3D roadmap throughout the procedure.
Overall, we judged that the fused 3D roadmap in this particular patient was subjectively useful but not essential. Arguably, the ring of the bioprosthesis could have been used as a sole marker for valve delivery. However, in our experience planning of the procedure with fusion software and later having a continuously available 3D roadmap enhances navigation of the abnormal enlarge right heart anatomy and selection of pulmonary artery branches for wire placement and subsequent introduction of the delivery system. This approach enables shortening of the diagnostic part of the procedure to hemodynamic measurements performed with low dose and low frame rate (3.5 per second) fluoroscopy. In selected cases, when accurate registration of the 3D roadmap is achieved, all angiographies prior to stent or valve implantation may be spared [9]. In this case, we performed 3DRA coronary testing despite the fixed ring of the prosthesis to determine whether high pressure balloon “cracking” of the failed valve would be safe to facilitate implantation of a larger Sapien valve. Coronary information obtained with 3DRA surpasses images available from fusion imaging or biplane angiography, and indisputably clarifies the relations between the RV outflow tract and the LCA. Moreover, the distance between the bioprosthesis ring and the LCA was measured more closely with the cross-sectional imaging analysis of the 3DRA than in the MRI measurements in the setting of the RV outflow deformation secondary to the stiff wire and the balloon inflated at the time of the spin. Selective coronary angiography in place of aortic root injection may risk underestimating ostial compression and/or ascending aorta deformation [3]. However, careful evaluation of the target structures and relationships during pre-processing of noninvasive 3D imaging with fusion software or during post-processing of 3DRA allows selection of those at risk, in whom aortic root injection should be performed. Simultaneous balloon inflation in the RV outflow leads to a decrease in cardiac output, resulting in less contrast washout for the aorta. Hence, there was no need to consider rapid pacing and a lower volume of contrast can be used. The 3D reconstruction of the balloon in RV outflow can be used as a roadmap with the indentation or a cutout slice marking the landing area for the valve. This was not necessary in the present case since we had the ring of the bioprosthesis available for guidance.
The unique feature of echocardiography, regardless of whether 2D or 3D, transthoracic, transesophageal or intra-cardiac, is that it provides dynamic images of the current anatomic situation, in contrast to fusion of pre-procedural 3D datasets or 3DRA, which provide static images [3, 6–9]. The drawbacks of ICE are the requirement of a relatively large vessel access, a single, relatively narrow plane of imaging for the 2D probe and the high cost. As we already had a large sheath in the vein, we used the access for introduction of the ICE probe. The obtained images enabled us to avoid the final angiography and coupled with hemodynamic data and electrocardiographic recordings reassured us of the good final outcome.
In this rapidly changing field we can look forward to the introduction of 3D ICE as well as the development of fusion overlay systems which predict and track vessel distortion caused by stiff delivery systems and respiratory movement. Although these may not produce appreciable differences in outcome for procedures such as that described above, they may boost operator confidence in fusion registration as well as facilitating applications such as coronary stenting and branch pulmonary artery interventions.
Safe and considered incorporation of multi-modality imaging can allow precise PPVI with minimal contrast use and radiation exposure. We contend that this minimalist approach is only appropriate where the ability to ramp up imaging is available in the event of complications.








