Extraction of the thrombo-occlusive material plays an important role in the interventional treatment of acute ischaemic stroke [1–3], acute myocardial infarction (AMI) [4], acute pulmonary artery embolism [5], and acute limb-threatening ischaemia [6]. Despite diminished interest in coronary thrombus extraction in AMI, thrombus aspiration has recently flourished in stroke intervention [7].
Today, the majority of thrombus evacuation technologies are based on, or include, aspiration [1–6]. The performance characteristics of aspiration catheters play a fundamental role in safely achieving a radiologically and clinically successful thrombus aspiration [1–7]. Aspiration catheters must balance (i) the maximal anatomically compatible inner diameter with (ii) active or passive aspiration to maintain the suction force, and (iii) the capability to accommodate and remove large thrombotic material volumes.
There is unequivocal evidence that thrombus fragmentation and distal embolisation – when primary angioplasty is performed in the absence of thrombus aspiration – may lead to a clinically-relevant increase in the infarct size [7]. Nevertheless, routine consideration of coronary thrombus aspiration in acute myocardial infarction has been clouded by misinterpretation of the data from 2 large randomised clinical trials suffering from major design flaws: TASTE and TOTAL [8–10]. It is regrettable that the guidelines have missed to consider the fundamental limitation of these 2 “landmark” trials.
Uncertainty as to the treatment effect (i.e. “needed” vs. “not needed”) is the fundamental principle of randomisation [11]. Unsurprisingly, the main publication from TASTE [8] lists “thrombus aspiration considered to be indicated” as a reason provided by the operators for not enrolling patients into the trial. This, while ethically commendable, makes the trial invalid for testing the efficacy of thrombus aspiration because the patients with an indication to thrombus aspiration were excluded from the trial and treated with thrombus aspiration outside the trial [9].
The other major trial, TOTAL, “randomly assigned 10,732 patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (PCI) to a strategy of routine upfront manual thrombectomy versus PCI alone”, irrespective of angiographic thrombus presence/absence [10]. Thus, the TOTAL patients were enrolled into the trial and received “thrombus aspiration” (if randomised to thrombus aspiration) irrespective of the presence/absence of indication to thrombus evacuation). While it is not surprising that thrombus aspiration would be ineffective in the absence of a thrombus, this critically confounds outcomes in the thrombus aspiration arm. Another “data killing effect” of the TOTAL trial is its inclusion of strokes occurring beyond 48 h after the procedure in the safety analysis of thrombus aspiration [10].
While the “drag-and-drop” effect seen occasionally with manual thrombus aspiration may lead to periprocedural stroke [4], there is no mechanistic explanation for any link between coronary thrombus aspiration and stroke occurrence at 30 or 180 days. Indeed, it is hard to comprehend how coronary thrombus aspiration on day 0 would relate to stroke between 48 h and 30 days post-procedure or between 48 h and 180 days post-procedure (sic!) as per the TOTAL study design [10].
One fundamental limitation of the coronary thrombus manual aspiration is the risk of “losing” the aspirated thrombus, particularly in case of the thrombus “hanging” at the tip of the aspiration catheter (“drag-and-drop” effect) [4]. In the absence of guiding catheter intubation into the coronary ostium and/or catheter malalignment, this may result in cerebral embolism [4]. Another significant contribution to the risk of stroke arises from the fundamental limitation of manual collect aspiration: it is suffering from decreasing (to absent) vacuum power as the aspiration is performed.
Analysis of the incidence stroke in the TOTAL trial showed, by 48 h, 15/5033 (0.30%) non-haemorrhagic strokes in the PCI + thrombectomy arm vs. 6/5033 (0.12%) strokes in the PCI-only arm; a difference neither statistically significant (95% CI: 0.97–6.44) [10] nor significant for clinical decision-making [11]. The “statistical significance” in stroke rate was driven by strokes occurring > 48 h post-procedure by 30 and 180 days [10]. In the absence of any (at least hypothetical) mechanistic link between thrombus aspiration on day 0 and a stroke between day 3 and day 180, and with a very low absolute event rate (35/5033 vs. 19/5033 by 30 days and 52/5033 vs. 29/5033 by 180 days) [10], the apparent difference in stroke incidence in the TOTAL trial has to be perceived as chance.
It is thus important to understand that neither the TASTE nor TOTAL trials showed any futility of routine consideration of thrombus aspiration in acute myocardial infarction, nor of performing it when indicated. The practice of evidence-based medicine means integrating the best available clinical evidence with individual expertise of the clinician [11]. Physicians have the right, and the duty, to critically interpretate the applicability of published data and their relevance in their treatment decision making [11].
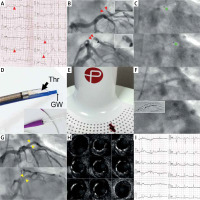
With our strategy of routine consideration of thrombus extraction in primary percutaneous interventions with angiographic thrombus evidence [8, 10], we present images (Figure 1) from our first-in-Poland use of a coronary thrombus aspiration system that employs neuro-tracking catheter technology and a mechanical vacuum aspiration system (constant aspiration, sustained aspiration). The neuro-tracking catheter technology and mechanical vacuum aspiration system have prior extensive evidence in cerebral thrombus contact evacuation efficacy [1, 12, 13]. While the unique design of the CAT RX catheter results in excellent navigability and the opportunity to reach more distal segments (as demonstrated in cerebral vessels whose anatomy is more challenging than coronary anatomy) [1, 12, 13], continuous vacuum pump aspiration translates into feasibility to remove large thrombus volumes with minimum risk of thrombus “drop” effect [1, 13]. These unique features make the system an attractive solution in the coronary contact thrombus aspiration armamentarium.
Figure 1
A typical example of thrombus-containing lesion in ST-segment elevation acute myocardial infarction (STEMI). Culprit lesion thrombus extraction using CAT RX contact aspiration system with sustained vacuum assistance. A – 12-lead electrocardiogram (ECG) in a 52-year-old patient, smoker, admitted after resuscitated cardiac arrest; note ST-segment elevations in antero-lateral leads (arrows). B – Emergency coronary angiography revealed a thrombotic lesion (arrows) in the intermediate branch (IM) of the left coronary artery; the flow was impaired (thrombolysis in myocardial infarction – TIMI grade 2). C – Consistent with totality of evidence [11], primary aspiration thrombectomy with stent placement intention was applied to minimise myocardial tissue loss. A single passage of the CAT RX (Penumbra) 6F mechanical vacuum-assisted coronary aspiration catheter system (tip marked with arrows, see also the photograph in D) resulted in evacuation of the thromboembolic lesion material (cf., E). D – Indigo CAT RX Neuro-Tracking Technology catheter for coronary thrombus aspiration (Thr – thrombus contact extraction lumen, GW – guidewire lumen); Penumbra Controlled Vacuum Engine filter is shown in E. E – Thrombus evacuated in this procedure (Penumbra Controlled Vacuum Engine filter in the background). F – Direct implantation (top) of a drug-eluting stent (Cruz 3.0 × 16 mm), note proximal stent edge in the ostial position; followed by non-compliant balloon (3.5 × 6 mm) sequential stent optimization (bottom, inflations up to 20 atm), ClearStent image in the inset. G – Final angiogram depicting an optimal angiographic result with TIMI 3 flow. H – Intravascular ultrasound run, showing optimal stent expansion and apposition in absence of any thrombotic material in the lumen – consistent with a safe and effective, complete thrombus extraction. I – 12-lead ECG performed immediately after procedure; note ST-segment regression by over 70% (consistent with effective myocardial tissue reperfusion). This was associated with a complete relief of chest pain and haemodynamic improvement
A recent 5-centre study of thrombus aspiration in the USA using the Indigo CAT RX Aspiration System in patients with AMI showed that it was safe and effective for reducing thrombus burden and restoring flow, in the absence of any cases of ischaemic stroke [14]. Similarly, in the ROPUST study (Cleveland) the CAT RX system provided safe and effective thrombus removal in high-risk patients with a large thrombus burden, in the absence of strokes or device-related complications [15]. Finally, in the prospective CHEETAH Study in 400 AMI patients with high thrombus burden, sustained mechanical aspiration using the CAT RX system was associated with high rates of thrombus extraction, flow restoration, and normal myocardial perfusion on final angiography. The 3 strokes by 30-days (0.77%) in CHEETAH, which occurred on days 2, 3, and 30, were independently adjudicated as being unrelated to the study device [16]. This is in line with our criticism of the erroneous interpretation of the data from the TASTE and TAPAS trials (see above). Indeed, a current review of interventional thrombus management in AMI points to recent meta-analyses demonstrating a reduction in cardiovascular death in patients with large thrombus burden randomly assigned to aspiration, as well as improvement in left ventricular function with thrombus aspiration in thrombus-containing lesions [7].
It remains to be decided whether the Indigo CAT RX continuous aspiration technology should be routinely used as the first-line approach (as in the procedure in Figure 1) to coronary thrombus aspiration or if it should be reserved – with a higher cost neuro-tracking technology catheter paired with a mechanical vacuum aspiration system – primarily for cases with high thrombus burden and/or failed manual thrombectomy [17].
In conclusion, coronary thrombus contact aspiration remains an important myocardial tissue-saving therapeutic modality in lesions with angiographic thrombus evidence. However, prior techniques of manual thrombus aspiration may fail to reach and/or remove the thrombotic material [4, 7, 8]. The CAT RX Indigo Continuous Aspiration System, employing neuro-tracking catheter technology for optimal catheter performance can offer a clinically-relevant improvement. Data should be collected for larger-scale analyses.








